G protein
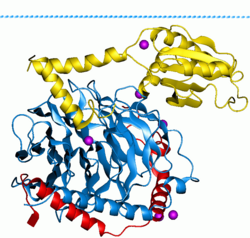
Phosducin- transducin beta-gamma complex. Beta and gamma subunits of G-protein are shown by blue and red, respectively.

Guanosine diphosphate

Guanosine triphosphate
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases.
There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein complexes. The latter class of complexes is made up of alpha (α), beta (β) and gamma (γ) subunits.[1] In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex
[2].
G proteins located within the cell are activated by G protein-coupled receptors (GPCRs) that span the cell membrane.[3] Signaling molecules bind to a domain of the GPCR located outside the cell, and an intracellular GPCR domain then in turn activates a particular G protein. Some inactive-state GPCRs have also been shown to be "pre-coupled" with G proteins.[4] The G protein activates a cascade of further signaling events that finally results in a change in cell function. G protein-coupled receptor and G proteins working together transmit signals from many hormones, neurotransmitters, and other signaling factors.[5] G proteins regulate metabolic enzymes, ion channels, transporter proteins, and other parts of the cell machinery, controlling transcription, motility, contractility, and secretion, which in turn regulate diverse systemic functions such as embryonic development, learning and memory, and homeostasis.[6]
.mw-parser-output .toclimit-2 .toclevel-1 ul,.mw-parser-output .toclimit-3 .toclevel-2 ul,.mw-parser-output .toclimit-4 .toclevel-3 ul,.mw-parser-output .toclimit-5 .toclevel-4 ul,.mw-parser-output .toclimit-6 .toclevel-5 ul,.mw-parser-output .toclimit-7 .toclevel-6 ul{display:none}
Contents
1 History
2 Function
3 Diversity
4 Signaling
4.1 Heterotrimeric
4.1.1 Common mechanism
4.1.1.1 Activation
4.1.1.2 Termination
4.1.2 Specific mechanisms
4.1.2.1 Gαs
4.1.2.2 Gαi
4.1.2.3 Gαq/11
4.1.2.4 Gα12/13
4.1.2.5 Gβ
4.2 Small GTPases
5 Lipidation
6 References
7 External links
History
G proteins were discovered when Alfred G. Gilman and Martin Rodbell investigated stimulation of cells by adrenaline. They found that when adrenaline binds to a receptor, the receptor does not stimulate enzymes (inside the cell) directly. Instead, the receptor stimulates a G protein, which then stimulates an enzyme. An example is adenylate cyclase, which produces the second messenger cyclic AMP.[7] For this discovery, they won the 1994 Nobel Prize in Physiology or Medicine.[8]
Nobel prizes have been awarded for many aspects of signaling by G proteins and GPCRs. These include receptor antagonists, neurotransmitters, neurotransmitter reuptake, G protein-coupled receptors, G proteins, second messengers, the enzymes that trigger protein phosphorylation in response to cAMP, and consequent metabolic processes such as glycogenolysis.
Prominent examples include (in chronological order of awarding):
- The 1947 Nobel Prize in Physiology or Medicine to Carl Cori, Gerty Cori and Bernardo Houssay, for their discovery of how glycogen is broken down to glucose and resynthesized in the body, for use as a store and source of energy. Glycogenolysis is stimulated by numerous hormones and neurotransmitters including adrenaline.
- The 1970 Nobel Prize in Physiology or Medicine to Julius Axelrod, Bernard Katz and Ulf von Euler for their work on the release and reuptake of neurotransmitters.
- The 1971 Nobel Prize in Physiology or Medicine to Earl Sutherland for discovering the key role of adenylate cyclase, which produces the second messenger cyclic AMP.[7]
- The 1988 Nobel Prize in Physiology or Medicine to George H. Hitchings, Sir James Black and Gertrude Elion "for their discoveries of important principles for drug treatment" targeting GPCRs.
- The 1992 Nobel Prize in Physiology or Medicine to Edwin G. Krebs and Edmond H. Fischer for describing how reversible phosphorylation works as a switch to activate proteins, and to regulate various cellular processes including glycogenolysis.[9]
- The 1994 Nobel Prize in Physiology or Medicine to Alfred G. Gilman and Martin Rodbell for their discovery of "G-proteins and the role of these proteins in signal transduction in cells".[10]
- The 2000 Nobel Prize in Physiology or Medicine to Eric Kandel, Arvid Carlsson and Paul Greengard, for research on neurotransmitters such as dopamine, which act via GPCRs.
- The 2004 Nobel Prize in Physiology or Medicine to Richard Axel and Linda B. Buck for their work on G protein-coupled olfactory receptors.[11]
- The 2012 Nobel Prize in Chemistry to Brian Kobilka and Robert Lefkowitz for their work on GPCR function.[12]
Function
G proteins are important signal transducing molecules in cells. "Malfunction of GPCR [G Protein-Coupled Receptor] signaling pathways are involved in many diseases, such as diabetes, blindness, allergies, depression, cardiovascular defects, and certain forms of cancer. It is estimated that about 30% of the modern drugs' cellular targets are GPCRs." [13] The human genome encodes roughly 800 [14]G protein-coupled receptors, which detect photons of light, hormones, growth factors, drugs, and other endogenous ligands. Approximately 150 of the GPCRs found in the human genome have still-unknown functions.
Whereas G proteins are activated by G protein-coupled receptors, they are inactivated by RGS proteins (for "Regulator of G protein signalling"). Receptors stimulate GTP binding (turning the G protein on). RGS proteins stimulate GTP hydrolysis (creating GDP, thus turning the G protein off).
Diversity
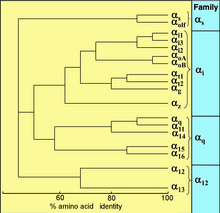
Sequence relationship among the 18 human Gα proteins.[15]
All eukaryotes use G proteins for signaling and has evolved a large diversity of G proteins. For instance, humans encode 18 different Gα proteins, 5 Gβ proteins, and 12 Gγ proteins.[15]
Signaling
G protein can refer to two distinct families of proteins. Heterotrimeric G proteins, sometimes referred to as the "large" G proteins, are activated by G protein-coupled receptors and are made up of alpha (α), beta (β), and gamma (γ) subunits. "Small" G proteins (20-25kDa) belong to the Ras superfamily of small GTPases. These proteins are homologous to the alpha (α) subunit found in heterotrimers, but are in fact monomeric, consisting of only a single unit. However, like their larger relatives, they also bind GTP and GDP and are involved in signal transduction.
Heterotrimeric
Different types of heterotrimeric G proteins share a common mechanism. They are activated in response to a conformational change in the GPCR, exchanging GDP for GTP, and dissociating in order to activate other proteins in a particular signal transduction pathway. The specific mechanisms, however, differ between protein types.
Common mechanism

Activation cycle of G-proteins (purple) by a G-protein-coupled receptor (GPCR, light blue) receiving a ligand (red). Ligand binding to GPCRs (2) induces a conformation change that facilitates the exchange of GDP for GTP on the α subunit of the heterotrimeric complex (3-4). Both GTP-bound Gα in the active form and the released Gβγ dimer can then go on to stimulate a number of downstream effectors (5). When the GTP on Gα is hydrolyzed to GDP (6) the original receptor is restored (1).[16]
Receptor-activated G proteins are bound to the inner surface of the cell membrane. They consist of the Gα and the tightly associated Gβγ subunits. There are many classes of Gα subunits: Gsα (G stimulatory), Giα (G inhibitory), Goα (G other), Gq/11α, and G12/13α are some examples. They behave differently in the recognition of the effector molecule, but share a similar mechanism of activation.
Activation
When a ligand activates the G protein-coupled receptor, it induces a conformational change in the receptor that allows the receptor to function as a guanine nucleotide exchange factor (GEF) that exchanges GDP for GTP – thus turning the GPCR "on". The GTP (or GDP) is bound to the Gα subunit in the traditional view of heterotrimeric GPCR activation. This exchange triggers the dissociation of the Gα subunit (which is bound to GTP) from the Gβγ dimer and the receptor as a whole. However, models which suggest molecular rearrangement, reorganization, and pre-complexing of effector molecules are beginning to be accepted.[4][17][18] Both Gα-GTP and Gβγ can then activate different signaling cascades (or second messenger pathways) and effector proteins, while the receptor is able to activate the next G protein.[19]
Termination
The Gα subunit will eventually hydrolyze the attached GTP to GDP by its inherent enzymatic activity, allowing it to re-associate with Gβγ and starting a new cycle. A group of proteins called Regulator of G protein signalling (RGSs), act as GTPase-activating proteins (GAPs), are specific for Gα subunits. These proteins accelerate the hydrolysis of GTP to GDP, thus terminating the transduced signal. In some cases, the effector itself may possess intrinsic GAP activity, which then can help deactivate the pathway. This is true in the case of phospholipase C-beta, which possesses GAP activity within its C-terminal region. This is an alternate form of regulation for the Gα subunit. Such Gα GAPs do not have catalytic residues (specific amino acid sequences) to activate the Gα protein. They work instead by lowering the required activation energy for the reaction to take place.[20]
Specific mechanisms
Gαs
Gαs activates the cAMP-dependent pathway by stimulating the production of cyclic AMP (cAMP) from ATP. This is accomplished by direct stimulation of the membrane-associated enzyme adenylate cyclase. cAMP can then act as a second messenger that goes on to interact with and activate protein kinase A (PKA). PKA can phosphorylate a myriad downstream targets.
The cAMP-dependent pathway is used as a signal transduction pathway for many hormones including:
ADH – Promotes water retention by the kidneys (created by the V2 cells of the posterior pituitary)
GHRH – Stimulates the synthesis and release of GH (somatotroph cells of the anterior pituitary)
GHIH – Inhibits the synthesis and release of GH (somatotroph cells of anterior pituitary)
CRH – Stimulates the synthesis and release of ACTH (anterior pituitary)
ACTH – Stimulates the synthesis and release of cortisol (zona fasiculata of the adrenal cortex in the adrenal glands)
TSH – Stimulates the synthesis and release of a majority of T4 (thyroid gland)
LH – Stimulates follicular maturation and ovulation in women; or testosterone production and spermatogenesis in men
FSH – Stimulates follicular development in women; or spermatogenesis in men
PTH – Increases blood calcium levels. This is accomplished via the Parathyroid hormone 1 receptor (PTH1) in the kidneys and bones, or via the Parathyroid hormone 2 receptor (PTH2) in the central nervous system and brain, as well as the bones and kidneys.
Calcitonin – Decreases blood calcium levels (via the calcitonin receptor in the intestines, bones, kidneys, and brain)
Glucagon – Stimulates glycogen breakdown in the liver
hCG – Promotes cellular differentiation, and is potentially involved in apoptosis.[citation needed]
Epinephrine – released by the adrenal medulla during the fasting state, when body is under metabolic duress. It stimulates glycogenolysis, in addition to the actions of glucagon.
Gαi
Gαi inhibits the production of cAMP from ATP.
Gαq/11
Gαq/11 stimulates the membrane-bound phospholipase C beta, which then cleaves PIP2 (a minor membrane phosphoinositol) into two second messengers, IP3 and diacylglycerol (DAG).
The Inositol Phospholipid Dependent Pathway is used as a signal transduction pathway for many hormones including:
- ADH (Vasopressin/AVP) – Induces the synthesis and release of glucocorticoids (Zona fasciculata of adrenal cortex in kidney); Induces vasoconstriction (V1 Cells of Posterior pituitary)
- TRH – Induces the synthesis and release of TSH (Anterior pituitary)
- TSH – Induces the synthesis and release of a small amount of T4 (Thyroid Gland)
- Angiotensin II – Induces Aldosterone synthesis and release (zona glomerulosa of adrenal cortex in kidney)
- GnRH – Induces the synthesis and release of FSH and LH (Anterior Pituitary)
Gα12/13
Gα12/13 are involved in Rho family GTPase signaling (see Rho family of GTPases). This is through the RhoGEF superfamily involving the RhoGEF domain of the proteins' structures). These are involved in control of cell cytoskeleton remodeling, and thus in regulating cell migration.
Gβ
- The Gβγ complexes sometimes also have active functions. Examples include coupling to and activating G protein-coupled inwardly-rectifying potassium channels.
Small GTPases
Small GTPases, also known as small G-proteins, bind GTP and GDP likewise, and are involved in signal transduction. These proteins are homologous to the alpha (α) subunit found in heterotrimers, but exist as monomers. They are small (20-kDa to 25-kDa) proteins that bind to guanosine triphosphate (GTP). This family of proteins is homologous to the Ras GTPases and is also called the Ras superfamily GTPases.
Lipidation
In order to associate with the inner leaflet[clarification needed] of the plasma membrane, many G proteins and small GTPases are lipidated, that is, covalently modified with lipid extensions. They may be myristolated, palmitoylated or prenylated.
References
^ Hurowitz EH, Melnyk JM, Chen YJ, Kouros-Mehr H, Simon MI, Shizuya H (April 2000). "Genomic characterization of the human heterotrimeric G protein alpha, beta, and gamma subunit genes". DNA Research. 7 (2): 111–20. doi:10.1093/dnares/7.2.111. PMID 10819326..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Clapham DE, Neer EJ (1997). "G protein beta gamma subunits". Annual Review of Pharmacology and Toxicology. 37: 167–203. doi:10.1146/annurev.pharmtox.37.1.167. PMID 9131251.
^ "Seven Transmembrane Receptors: Robert Lefkowitz". 2012-09-09. Retrieved 2016-07-11.
^ ab Qin K, Dong C, Wu G, Lambert NA (August 2011). "Inactive-state preassembly of G(q)-coupled receptors and G(q) heterotrimers". Nature Chemical Biology. 7 (10): 740–7. doi:10.1038/nchembio.642. PMC 3177959. PMID 21873996.
^ Reece J, C N (2002). Biology. San Francisco: Benjamin Cummings. ISBN 0-8053-6624-5.
^ Neves SR, Ram PT, Iyengar R (May 2002). "G protein pathways". Science. 296 (5573): 1636–9. Bibcode:2002Sci...296.1636N. doi:10.1126/science.1071550. PMID 12040175.
^ ab The Nobel Prize in Physiology or Medicine 1994, Illustrated Lecture.
^ Press Release: The Nobel Assembly at the Karolinska Institute decided to award the Nobel Prize in Physiology or Medicine for 1994 jointly to Alfred G. Gilman and Martin Rodbell for their discovery of "G-proteins and the role of these proteins in signal transduction in cells". 10 October 1994
^ "The Nobel Prize in Physiology or Medicine 1992 Press Release". Nobel Assembly at Karolinska Institutet. Retrieved 21 August 2013.
^ Press Release
^ "Press Release: The 2004 Nobel Prize in Physiology or Medicine". Nobelprize.org. Retrieved 8 November 2012.
^ Royal Swedish Academy of Sciences (10 October 2012). "The Nobel Prize in Chemistry 2012 Robert J. Lefkowitz, Brian K. Kobilka". Retrieved 10 October 2012.
^ Bosch DE, Siderovski DP (March 2013). "G protein signaling in the parasite Entamoeba histolytica". Experimental & Molecular Medicine. 45 (1038): e15. doi:10.1038/emm.2013.30. PMC 3641396. PMID 23519208.
^ Baltoumas FA, Theodoropoulou MC, Hamodrakas SJ (June 2013). "Interactions of the α-subunits of heterotrimeric G-proteins with GPCRs, effectors and RGS proteins: a critical review and analysis of interacting surfaces, conformational shifts, structural diversity and electrostatic potentials". Journal of Structural Biology. 182 (3): 209–18. doi:10.1016/j.jsb.2013.03.004. PMID 23523730.
^ ab Syrovatkina V, Alegre KO, Dey R, Huang XY (September 2016). "Regulation, Signaling, and Physiological Functions of G-Proteins". Journal of Molecular Biology. 428 (19): 3850–68. doi:10.1016/j.jmb.2016.08.002. PMC 5023507. PMID 27515397.
^ Stewart, Adele; Fisher, Rory A. (2015). Progress in Molecular Biology and Translational Science. Elsevier. pp. 1–11. doi:10.1016/bs.pmbts.2015.03.002. ISBN 9780128029381.
^ Digby GJ, Lober RM, Sethi PR, Lambert NA (November 2006). "Some G protein heterotrimers physically dissociate in living cells". Proceedings of the National Academy of Sciences of the United States of America. 103 (47): 17789–94. Bibcode:2006PNAS..10317789D. doi:10.1073/pnas.0607116103. PMC 1693825. PMID 17095603.
^ Khafizov K, Lattanzi G, Carloni P (June 2009). "G protein inactive and active forms investigated by simulation methods". Proteins. 75 (4): 919–30. doi:10.1002/prot.22303. PMID 19089952.
^ Yuen JW, Poon LS, Chan AS, Yu FW, Lo RK, Wong YH (June 2010). "Activation of STAT3 by specific Galpha subunits and multiple Gbetagamma dimers". The International Journal of Biochemistry & Cell Biology. 42 (6): 1052–9. doi:10.1016/j.biocel.2010.03.017. PMID 20348012.
^ Sprang SR, Chen Z, Du X (2007). "Structural basis of effector regulation and signal termination in heterotrimeric Galpha proteins". Advances in Protein Chemistry. Advances in Protein Chemistry. 74: 1–65. doi:10.1016/S0065-3233(07)74001-9. ISBN 978-0-12-034288-4. PMID 17854654.
External links
| Wikimedia Commons has media related to G proteins. |
GTP-Binding Proteins at the US National Library of Medicine Medical Subject Headings (MeSH)