Cucurbitane
 | |
| Identifiers | |
|---|---|
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChemSpider |
|
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C30H54 |
Molar mass | 7002414762000000000♠414.762 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Cucurbitane is a chemical compound with formula C
30H
54 (CAS number 65441-59-0). It is a polycyclic hydrocarbon, specifically a triterpene. It is an isomer of lanostane (specifically 19(10→9β)-abeolanostane), from which it differs by the formal shift of a methyl group (carbon number 19) from the 10 to the 9β position in the standard steroid numbering scheme.[1][2]
The name is applied to two stereoisomers, distinguished by the prefixes 5α- and 5β-, which differ by the handedness of the bonds at a particular carbon atom (number 5 in the standard steroid numbering scheme).[1]
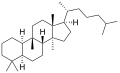
5α-Cucurbitane
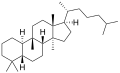
5β-Cucurbitane
Contents
1 Derivatives
1.1 Natural compounds
1.1.1 Named
1.1.2 Unnamed
2 See also
3 References
Derivatives
Natural compounds
Compounds with the basic cucurbitane skeleton are found in many plants, and some are important phytopharmaceuticals,[3] Natural cucurbitane-related compounds include:
Named
Balsaminapentaol, from Momordica balsamina.[4]
Balsaminol A, from Momordica balsamina.[4]
Balsaminol B, from Momordica balsamina.[4]
Brydioside A from Bryonia dioica [3]:64
Bryoamaride and derivatives from Bryonia dioica [3]:65,66
Charantin or foetidin, from Momordica charantia[5] and Momordica foetida[6]
Charantosides I-VIII, from Momordica charantia.[7]
Cucurbalsaminol B, from Momordica balsamina.[4]
Cucurbalsaminol A, from Momordica balsamina.[4]
Cucurbitacins A-L, O-T [3][8][9]:3–8
Datiscosides, from Datisca glomerata [3]:16–19
Endecaphyllacins A and B, from roots of Hemsleya endecaphylla [9]:1,2
Hemslecins A and B, from roots of H. endecaphylla [9]
Lepidolide, from the mushroom Russula lepida [10]
Karavilagenin E, from Momordica balsamina.[4]
Khekadaengosides A, B, D and K, from Trichosanthes tricuspidata [3]:57,58,67,68
Kuguacins A-S, from stems and leaves of Momordica charantia[11][12]
Kuguaglycosides A-H, from the root of Momordica charantia[13]
Mogrosides I-V, from the fruits of Siraitia grosvenorii [14]
Momordicin I, II and 28, from Momordica charantia[15][16]
Momordicines II and IV, from leaves of Momordica charantia [17]
Momordicosides A-S, from Momordica charantia fruits [7][18][19]
Neokuguaglucoside, from Momordica charantia fruits [20]
Neomogroside, from the fruit of Siraitia grosvenorii.[21]
Pentanorcucurbitacins A and B [22]:1,2
Perseapicroside A, from Persea mexicana [3]:44
Scandenoside R9, from Hemsleya panacis-scandens [3]:45
Spinosides A and B, from Desfontainia spinosa [3]:61,62
Unnamed
3β,7β,23ξ-trihydroxycucurbita-5,24-dien-19-al, soluble in chloroform, melts at 123−125 °C, from Momordica charantia, Momordica foetida.[23]:1
3β,7β,25-trihydroxycucurbita-5,23-dien-19-al, soluble in chloroform, melts at 188−191 °C, from Momordica charantia, Momordica foetida[23]:2
3β,7β-dihydroxy-25-methoxycucurbita-5,23-dien-19-al, soluble in chloroform, from Momordica charantia, Momordica foetida[23]:3
5β,19-epoxy-25-methoxycucurbita-6,23-dien-3β,19-diol, soluble in chloroform, melts at 182−184 °C, from Momordica foetida[23]:4
5β,19-epoxycucurbita-6,23-dien-3β,19,25-triol, soluble in chloroform, from Momordica foetida[23]:5
5β,19-epoxy-19-methoxycucurbita-6,23-dien-3β,25-diol, soluble in chloroform, melts at 102−104 °C, from Momordica charantia, Momordica foetida[23]:6
5β,19-epoxy-19,25-dimethoxycucurbita-6,23-dien-3β-ol, soluble in chloroform, from Momordica charantia, Momordica foetida[23]:7
5β,19-epoxy-25-methoxycucurbita-6,23-dien-3β-ol, soluble in chloroform, melts at 139−141 °C, from Momordica charantia, Momordica foetida[23]:8
19(R)-n-butanoxy-5β,19-epoxycucurbita-6,23-diene-3β,25-diol 3-O-β-glucopyranoside, C
40H
66O
9, white powder soluble in methanol, from Momordica charantia fruit (8 mg/35 kg)[18]:1
23-O-β-allopyranosylecucurbita-5,24-dien-7α,3β,22(R),23(S)-tetraol 3-O-β-allopyranoside,C
42H
69O
14, white powder soluble in methanol, from Momordica charantia fruit (10 mg/35 kg)[18]:2
23(R),24(S),25-trihydroxycucurbit-5-ene 3-O-{[β-glucopyranosyl(1→6)]-O-β-glucopyranosyl}-25-O-β-glucopyranoside, C
48H
82O
19, white powder soluble in methanol, from Momordica charantia fruit (10 mg/35 kg)[18]:3
2,16-dihydroxy-22,23,24,25,26,27-hexanorcucurbit-5-en-11,20-dione 2-O-β-D-glucopyranoside, soluble in ethanol, from Cucurbita pepo fruits (25 mg/15 kg) [8]:3
16-hydroxy-22,23,24,25,26,27-hexanorcucurbit-5-en-11,20-dione 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside, white powder, soluble in ethanol, from Cucurbita pepo fruits (12 mg/15 kg)[8]:4
7-methoxycucurbita-5,24-diene-3β,23(R)-diol, from Momordica balsamina [24]
25,26,27-trinorcucurbit-5-ene-3,7,23-trione C
27H
40O
3, white powder, soluble in methanol, from stems of Momordica charantia (6 mg/18 kg)[22]:3
See also
- Goyaglicoside
- Karaviloside
Momordenol, from Momordica charantia[15]
24(R)-stigmastan-3β,5α,6β-triol-25-ene 3-O-β-glucopyranoside, C
35H
60O
8, white powder, from Momordica charantia fruit (15 mg/35 kg)[18]:4
References
^ ab IUPAC Commission on the Nomenclature of Organic Chemistry and IUPAC-IUB Commission on Biochemical Nomenclature (1969), The Nomenclature of Steroids — Revised Tentative Rules. European Journal of Biochemistry, volume 10, 1-19
^ Satish Kumar and Raj Kumar (1991), Dictionary of Biochemistry. Anmol Publications, India
^ abcdefghi Chen, J. C.; Chiu, M. H.; Nie, R. L.; Cordell, G. A.; Qiu, S. X. (2005). "Cucurbitacins and cucurbitane glycosides: Structures and biological activities". Natural Product Reports. 22 (3): 386–399. doi:10.1039/B418841C. PMID 16010347..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ abcdef Ramalhete, C. T.; Mansoor, T. A.; Mulhovo, S.; Molnár, J.; Ferreira, M. J. U. (2009). "Cucurbitane-Type Triterpenoids from the African PlantMomordica balsamina". Journal of Natural Products. 72 (11): 2009–2013. doi:10.1021/np900457u. PMID 19795842.
^ M. M. Lolitkar and M. R. Rajarama Rao (1962), "Note on a Hypoglycaemic Principle Isolated from the fruits of Momordica charantia". Journal of the University of Bombay, volume 29, pages 223-224
^ Olaniyi, A. A. (1975). "A neutral constituent of Momordica foetida". Lloydia. 38 (4): 361–362. PMID 1186439.
^ ab Akihisa, T.; Higo, N.; Tokuda, H.; Ukiya, M.; Akazawa, H.; Tochigi, Y.; Kimura, Y.; Suzuki, T.; Nishino, H. (2007). "Cucurbitane-Type Triterpenoids from the Fruits of Momordica charantia and Their Cancer Chemopreventive Effects". Journal of Natural Products. 70 (8): 1233–1239. doi:10.1021/np068075p. PMID 17685651.
^ abc Da-Cheng Wang, Hong-Yu Pan, Xu-Ming Deng, Hua Xiang, Hui-Yuan Gao, Hui Cai, and Li-Jun Wu (2007), "Cucurbitane and hexanorcucurbitane glycosides from the fruits of Cucurbita pepo cv dayangua". Journal of Asian Natural Products Research, volume 9, issue 6, pages 525–529.
^ abc Chen, J. C.; Zhang, G. H.; Zhang, Z. Q.; Qiu, M. H.; Zheng, Y. T.; Yang, L. M.; Yu, K. B. (2008). "Octanorcucurbitane and Cucurbitane Triterpenoids from the Tubers ofHemsleya endecaphyllawith HIV-1 Inhibitory Activity". Journal of Natural Products. 71 (1): 153–155. doi:10.1021/np0704396. PMID 18088099.
^ Jian-Wen Tan, Ze-Jun Dong, Zhi-Hui Ding and Ji-Kai Liu (2002), "Lepidolide, a Novel Seco-ring-A Cucurbitane Triterpenoid from Russula lepida (Basidiomycetes)". Zeitschrift für Naturforschung Series C, volume 57C issue 11/12, pages 963-965.
^ Chen, J. C.; Liu, W. Q.; Lu, L.; Qiu, M. H.; Zheng, Y. T.; Yang, L. M.; Zhang, X. M.; Zhou, L.; Li, Z. R. (2009). "Kuguacins F–S, cucurbitane triterpenoids from Momordica charantia". Phytochemistry. 70 (1): 133–140. doi:10.1016/j.phytochem.2008.10.011. PMID 19041990.
^ J. C. Chen, R. R. Tian, M. H. Qiu, L. Lu, Y. T. Zheng, Z. Q. Zhang (2008), "Trinorcucurbitane and cucurbitane triterpenoids from the roots of Momordica charantia." Phytochemistry, volume 69, pages 1043–1048
^ Chen, J. C.; Lu, L.; Zhang, X. M.; Zhou, L.; Li, Z. R.; Qiu, M. H. (2008). "Eight New Cucurbitane Glycosides, Kuguaglycosides A – H, from the Root ofMomordica charantia L". Helvetica Chimica Acta. 91 (5): 920. doi:10.1002/hlca.200890097.
^ Midori Takasaki, Takao Konoshima, Yuji Murata, Masaki Sugiura, Hoyoku Nishino, Harukuni Tokuda, Kazuhiro Matsumoto, Ryoji Kasai, and Kazuo Yamasaki (2003) "Anticarcinogenic activity of natural sweeteners, cucurbitane glycosides, from Momordica grosvenori". Cancer Letters, volume 198, pages 37–42
^ ab Sabira Begum, Mansour Ahmed, Bina S. Siddiqui, Abdullah Khan, Zafar S. Saify, and Mohammed Arif (1997), "Triterpenes, A Sterol and a Monocyclic Alcohol From Momordica Charantia." Phytochemistry, volume 44, issue 7, pages 1313-1320
^ Majekodunmi Fatope, Yoshio Takeda, Hiroyasu Yamashita, Hikaru Okabe, and Tatsuo Yamauchi (1990), "New cucurbitane trirterpenoids from Momordica charantia." Journal of Natural Products, volume 53, issue 6, pages 1491-1497.
^ Daniel Bisrat Mekuria, Takehiro Kashiwagi, Shin-ichi Tebayashi, and Chul-Sa Kim (2006)"Cucurbitane Glucosides from Momordica charantia Leaves as Oviposition Deterrents to the Leafminer, Liriomyza trifolii". Z. Naturforsch., volume 61c, pages 81–86
^ abcde Liu, J. Q.; Chen, J. C.; Wang, C. F.; Qiu, M. H. (2009). "New Cucurbitane Triterpenoids and Steroidal Glycoside from Momordica charantia". Molecules. 14 (12): 4804–4813. doi:10.3390/molecules14124804. PMID 20032860.
^ Harinantenaina, L.; Tanaka, M.; Takaoka, S.; Oda, M.; Mogami, O.; Uchida, M.; Asakawa, Y. (2006). "Momordica charantia Constituents and Antidiabetic Screening of the Isolated Major Compounds". Chemical & Pharmaceutical Bulletin. 54 (7): 1017. doi:10.1248/cpb.54.1017.
^ Liu, J. Q.; Chen, J. C.; Wang, C. F.; Qiu, M. H. (2010). "One new cucurbitane triterpenoid from the fruits of Momordica charantia". European Journal of Chemistry. 1 (4): 294. doi:10.5155/eurjchem.1.4.294-296.131.
^ Si Jian-yong, Chen Di-hua, Chang Qi and Shen Lian-gang (1996), Isolation and Determination of Cucurbitane-Glycosides from Fresh Fruits of Siraitia Grosvenorii. Journal of Integrative Plant Biology, volume 38, issue 6, pages, 489–494
^ ab Chen, C. R.; Liao, Y. W.; Wang, L.; Kuo, Y. H.; Liu, H. J.; Shih, W. L.; Cheng, H. L.; Chang, C. I. (2010). "Cucurbitane Triterpenoids from Momordica charantia and Their Cytoprotective Activity in tert-Butyl Hydroperoxide-Induced Hepatotoxicity of HepG2 Cells". Chemical & Pharmaceutical Bulletin. 58 (12): 1639. doi:10.1248/cpb.58.1639.
^ abcdefgh Mulholland, D. A.; Sewram, V.; Osborne, R.; Pegel, K. H.; Connolly, J. D. (1997). "Cucurbitane triterpenoids from the leaves of Momordica foetida". Phytochemistry. 45 (2): 391. doi:10.1016/S0031-9422(96)00814-X.
^ Gabriella Spengler, Cátia Ramalhete, Marta Martins, Ana Martins, Julianna Serly, Miguel Viveiros, Joseph Molnár, Noélia Duarte, Silva Mulhovos, Maria-José U. Ferreira, Leonard Amaral (2010), "Evaluation of Cucurbitane-type Triterpenoids from Momordica balsamina on P-Glycoprotein (ABCB1) by Flow Cytometry and Real-time Fluorometry", Anticancer Research, volume 30, pages 4867-4871

