Purinergic receptor
| Part of a series on |
| Purinergic signalling |
|---|
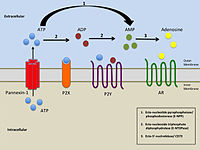 Simplified illustration of extracellular purinergic signalling |
Concepts |
Purinergic signalling
|
Membrane transporters |
Nucleoside transporters
|
Purinergic receptors, also known as purinoceptors, are a family of plasma membrane molecules that are found in almost all mammalian tissues.[1] Within the field of purinergic signalling, these receptors have been implicated in learning and memory, locomotor and feeding behavior, and sleep.[2] More specifically, they are involved in several cellular functions, including proliferation and migration of neural stem cells, vascular reactivity, apoptosis and cytokine secretion.[2][3] These functions have not been well characterized and the effect of the extracellular microenvironment on their function is also poorly understood.
The term purinergic receptor was originally introduced to illustrate specific classes of membrane receptors that mediate relaxation of gut smooth muscle as a response to the release of ATP (P2 receptors) or adenosine (P1 receptors). P2 receptors have further been divided into five subclasses: P2X, P2Y, P2Z, P2U, and P2T. To distinguish P2 receptors further, the subclasses have been divided into families of metabotropic (P2Y, P2U, and P2T) and ionotropic receptors (P2X and P2Z).[4]
In 2014, the first purinergic receptor in plants, DORN1, was discovered.[5]
Contents
1 3 classes of purinergic receptors
2 P2X receptors
3 P2Y and P1 receptors
4 Inhibitors
5 Effects on chronic pain
6 Effects on cytotoxic edema
7 Effects on diabetes
8 See also
9 References
10 External links
3 classes of purinergic receptors
| Name | Activation | Class |
| P1 receptors | adenosine | G protein-coupled receptors |
| P2Y receptors | nucleotides
| G protein-coupled receptors |
| P2X receptors | ATP | ligand-gated ion channel |
There are three known distinct classes of purinergic receptors, known as P1, P2X, and P2Y receptors. [What about P2Z,U,T?]
P2X receptors
P2X receptors are ligand-gated ion channels, whereas the P1 and P2Y receptors are G protein-coupled receptors. These ligand-gated ion channels are nonselective cation channels responsible for mediating excitatory postsynaptic responses, similar to nicotinic and ionotropic glutamate receptors.[6] P2X receptors are distinct from the rest of the widely known ligand-gated ion channels, as the genetic encoding of these particular channels indicates the presence of only two transmembrane domains within the channels.[1] These receptors are greatly distributed in neurons and glial cells throughout the central and peripheral nervous systems.[1] P2X receptors mediate a large variety of responses including fast transmission at central synapses, contraction of smooth muscle cells, platelet aggregation, macrophage activation, and apoptosis.[2][7] Moreover, these receptors have been implicated in integrating functional activity between neurons, glial, and vascular cells in the central nervous system, thereby mediating the effects of neural activity during development, neurodegeneration, inflammation, and cancer.[2]
P2Y and P1 receptors
Both of these metabotropic receptors are distinguished by their reactivity to specific activators. P1 receptors are preferentially activated by adenosine and P2Y receptors are preferentially more activated by ATP. P1 and P2Y receptors are known to be widely distributed in the brain, heart, kidneys, and adipose tissue. Xanthines (e.g. caffeine) specifically block adenosine receptors, and are known to induce a stimulating effect to one's behavior.[8]
Inhibitors
Inhibitors of purinergic receptors include clopidogrel, prasugrel and ticlopidine, as well as ticagrelor. All of these are antiplatelet agents that block P2Y12 receptors.
Effects on chronic pain
Data obtained from using P2 receptor-selective antagonists has produced evidence supporting ATP's ability to initiate and maintain chronic pain states after exposure to noxious stimuli. It is believed that ATP functions as a pronociceptive neurotransmitter, acting at specific P2X and P2Y receptors in a systemized manner, which ultimately (as a response to noxious stimuli) serve to initiate and sustain heightened states of neuronal excitability. This recent knowledge of purinergic receptors' effects on chronic pain provide promise in discovering a drug that specifically targets individual P2 receptor subtypes. While some P2 receptor-selective compounds have proven useful in preclinical trials, more research is required to understand the potential viability of P2 receptor antagonists for pain.[9]
Recent research has identified a role for microglial P2X receptors in neuropathic pain and inflammatory pain, especially the P2X4 and P2X7 receptors.[10][11][12][13][14]
Effects on cytotoxic edema
Purinergic receptors have been suggested to play a role in the treatment of cytotoxic edema and brain infarctions. It was found that with treatment of the purinergic ligand 2-methylthioladenosine 5' diphosphate (2-MeSADP), which is an agonist and has a high preference for the purinergic receptor type 1 isoform (P2Y1R), significantly contributes to the reduction of an ischemic lesions caused by cytotoxic edema. Further pharmacological evidence has suggested that 2MeSADP protection is controlled by enhanced astrocyte mitochondrial metabolism through increased inositol triphosphate-dependent calcium release. There is evidence suggesting a relationship between the levels of ATP and cytotoxic edema, where low ATP levels are associated with an increased prevalence of cytotoxic edema. It is believed that mitochondria play an essential role in the metabolism of astrocyte energy within the penumbra of ischemic lesions. By enhancing the source of ATP provided by mitochondria, there could be a similar 'protective' effect for brain injuries in general.[15]
Effects on diabetes
Purinergic receptors have been implicated in the vascular complications associated with diabetes due to the effect of high-glucose concentration on ATP-mediated responses in human fibroblasts.[16]
See also
- Purinergic signaling
References
^ abc North RA (Oct 2002). "Molecular physiology of P2X receptors". Physiological Reviews. 82 (4): 1013–67. doi:10.1152/physrev.00015.2002. PMID 12270951..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ abcd Burnstock, G. (2013). "Introduction to Purinergic Signalling in the Brain". Glioma Signaling. Advances in Experimental Medicine and Biology. 986. pp. 1–12. doi:10.1007/978-94-007-4719-7_1. ISBN 978-94-007-4718-0. PMID 22879061.
^ Ulrich H, Abbracchio MP, Burnstock G (Sep 2012). "Extrinsic purinergic regulation of neural stem/progenitor cells: implications for CNS development and repair". Stem Cell Reviews. 8 (3): 755–67. doi:10.1007/s12015-012-9372-9. PMID 22544361.
^ King BF, Burnstock G (2002) Purinergic receptors. In: Pangalos M, Davies C (eds) Understanding G protein-coupled receptors and their role in the CNS. Oxford University Press, Oxford, pp 422– 438
^ Cao Y, Tanaka K, Nguyen CT, Stacey G (Aug 2014). "Extracellular ATP is a central signaling molecule in plant stress responses". Current Opinion in Plant Biology. 20: 82–7. doi:10.1016/j.pbi.2014.04.009. PMID 24865948.
^ Kaczmarek-Hájek K, Lörinczi E, Hausmann R, Nicke A (Sep 2012). "Molecular and functional properties of P2X receptors--recent progress and persisting challenges". Purinergic Signalling. 8 (3): 375–417. doi:10.1007/s11302-012-9314-7. PMC 3360091. PMID 22547202.
^ Burnstock G, Fredholm BB, North RA, Verkhratsky A (Jun 2010). "The birth and postnatal development of purinergic signalling". Acta Physiologica. 199 (2): 93–147. doi:10.1111/j.1748-1716.2010.02114.x. PMID 20345419.
^ Neuroscience. 2nd edition.
Purves D, Augustine GJ, Fitzpatrick D, et al., editors.
Sunderland (MA): Sinauer Associates; 2001.
^ M.F. Jarvis
The neural–glial purinergic receptor ensemble in chronic pain states
Trends Neurosci., 33 (2010), pp. 48–57
^ Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K (2009). "Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays". Molecular Pain. 5: 1744–8069–5–28. doi:10.1186/1744-8069-5-28. PMC 2704200. PMID 19515262.
^ Ulmann L, Hirbec H, Rassendren F (Jul 2010). "P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain". The EMBO Journal. 29 (14): 2290–300. doi:10.1038/emboj.2010.126. PMC 2910276. PMID 20562826.
^ Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K (Aug 2003). "P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury". Nature. 424 (6950): 778–83. doi:10.1038/nature01786. PMID 12917686.
^ Kobayashi K, Takahashi E, Miyagawa Y, Yamanaka H, Noguchi K (Oct 2011). "Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model". Neuroscience Letters. 504 (1): 57–61. doi:10.1016/j.neulet.2011.08.058. PMID 21924325.
^ Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN (Apr 2005). "Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain". Pain. 114 (3): 386–96. doi:10.1016/j.pain.2005.01.002. PMID 15777864.
^ Zheng W, Watts LT, Holstein DM, Prajapati SI, Keller C, et al. (2010) Purinergic Receptor Stimulation Reduces Cytotoxic Edema and Brain Infarcts in Mouse Induced by Photothrombosis by Energizing Glial Mitochondria. PLoS ONE 5(12): e14401. doi:10.1371/journal.pone.0014401
^ Solini A, Chiozzi P, Falzoni S, Morelli A, Fellin R, Di Virgilio F (Oct 2000). "High glucose modulates P2X7 receptor-mediated function in human primary fibroblasts". Diabetologia. 43 (10): 1248–56. doi:10.1007/s001250051520. PMID 11079743.
External links
- IUPHAR GPCR Database - Adenosine receptors
- IUPHAR GPCR Database - P2Y receptors
Purinergic+Receptors at the US National Library of Medicine Medical Subject Headings (MeSH)