c-Raf
| RAF1 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||
| Aliases | RAF1, Raf-1 proto-oncogene, serine/threonine kinase, CMD1NN, CRAF, NS5, Raf-1, c-Raf | ||||||||||||||||||||||||
| External IDs | OMIM: 164760 MGI: 97847 HomoloGene: 48145 GeneCards: RAF1 | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Human | Mouse | |||||||||||||||||||||||
| Entrez |
|
| |||||||||||||||||||||||
| Ensembl |
|
| |||||||||||||||||||||||
| UniProt |
|
| |||||||||||||||||||||||
| RefSeq (mRNA) |
|
| |||||||||||||||||||||||
| RefSeq (protein) |
|
| |||||||||||||||||||||||
| Location (UCSC) | n/a | Chr 6: 115.62 – 115.68 Mb | |||||||||||||||||||||||
PubMed search | [2] | [3] | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
RAF proto-oncogene serine/threonine-protein kinase, also known as proto-oncogene c-RAF or simply c-Raf or even Raf-1, is an enzyme[4] that in humans is encoded by the RAF1 gene.[5][6] The c-Raf protein is part of the ERK1/2 pathway as a MAP kinase kinase kinase (MAP3K) that functions downstream of the Ras subfamily of membrane associated GTPases.[7] C-Raf is a member of the Raf kinase family of serine/threonine-specific protein kinases, from the TKL (Tyrosine-kinase-like) group of kinases.
Contents
1 Discovery
2 Structure
3 Evolutionary relationships
4 Regulation of activity
5 Associated human diseases
6 Role in cancer
6.1 B-Raf mutations
7 As a therapeutic target
8 List of interacting proteins
9 See also
10 References
11 Further reading
12 External links
Discovery
The first Raf gene, v-Raf was found in 1983. It was isolated from the murine retrovirus bearing the number 3611. It was soon demonstrated to be capable to transform rodent fibroblasts to cancerous cell lines, so this gene was given the name Virus-induced Rapidly Accelerated Fibrosarcoma (V-RAF).[5] A year later, another transforming gene was found in the avian retrovirus MH2, named v-Mil - that turned out to be highly similar to v-Raf.[8] Researchers were able to demonstrate that these genes encode enzymes that have serine-threonine kinase activity.[9] Normal cellular homologs of v-Raf and v-Mil were soon found in both the mouse and chicken genome (hence the name c-Raf for the normal cellular Raf gene), and it became clear that these too had a role in regulating growth and cell division.[10][11] Now we know that c-Raf is a principal component of the first described mitogen-activated protein kinase (MAPK) pathway: ERK1/2 signaling.[12] It acts as a MAP3 kinase, initiating the entire kinase cascade. Subsequent experiments showed that the normal, cellular Raf genes can also mutate to become oncogenes, by "overdriving" MEK1/2 and ERK1/2 activity.[13] In fact, vertebrate genomes contain multiple Raf genes. Several years later after the discovery of c-Raf, two further related kinases were described: A-Raf and B-Raf. The latter became the focus of research in recent years, since a large portion of human tumors carry oncogenic 'driver' mutations in the B-Raf gene.[14] These mutations induce an uncontrolled, high activity of Raf enzymes. Thus diagnostic and therapeutic interest in Raf kinases reached a new peak in the recent years.[15]
Structure
The human c-Raf gene is located on chromosome 3. At least two isoforms of mRNA have been described (arising from inclusion or removal of an alternative exon) that display only minute differences. The shorter, major isoform - consisting of 17 exons - encodes a protein kinase of 648 amino acids.[16]

A schematic architecture of human c-Raf protein
Similarly to many other MAPKKKs, c-Raf is a multidomain protein, with several additional domains to aid the regulation of its catalytic activity. On its N-terminal segment, a Ras-binding domain (RBD) and a C-kinase homologous domain 1 (C1 domain) are found next to each other. Structures of both conserved domains were solved in the past decades, shedding light on the mechanisms of their regulation.
The Ras-binding domain displays a ubiquitin-like fold (like many other small G-protein associating domains) and selectively binds GTP-bound Ras proteins only.[17][18][19] (You can see this interaction in high detail in the PDB box attached to the article. It shows Rap1 in complex with the RBD of c-Raf.)
The C1 domain - immediately downstream of the Ras binding domain - is a special zinc finger, rich in cysteines and stabilized by two zinc ions. It is similar to the diacylglycerol-binding C1 domains of protein kinase C (PKC) enzymes.[20][21] But unlike PKC, the C1 domains of Raf family kinases do not bind diacylglycerol.[22] Instead, they interact with other lipids, such as ceramide[22] or phosphatidic acid,[23] and even aid in the recognition of activated Ras (GTP-Ras).[21][24]
The close proximity of these two domains as well as several lines of experimental data suggest that they act as a single unit to negatively regulate the activity of the protein kinase domain, by direct physical interaction.[25] Historically, this autoinhibitory block was labelled as the CR1 region ("Conserved Region 1"), the hinge region being named CR2, and the kinase domain CR3. Unfortunately, the precise structure of the autoinhibited kinase remains unknown.
Between the autoinhibitory domain block and the catalytic kinase domain, a long segment - characteristic to all Raf proteins - can be found. It is highly enriched in serine amino acids, but its precise sequence is poorly conserved across related Raf genes. This region appears to be intrinsically unstructured, and very flexible. Its most likely role is to act as a natural "hinge" between the rigidly folded autoinhibitory and catalytic domains, enabling complex movements and profound conformational rearrangements within the molecule.[26] This hinge region contains a small, conserved island of amino acids, that are responsible for 14-3-3 protein recognition, but only when a critical serine (Ser259 in human c-Raf) is phosphorylated. A second, similar motif is found on the extreme C-terminus (centered around the phosphorylatable Ser 621) of all Raf enzymes, but downstream of the kinase domain.
The C-terminal half of c-Raf folds into a single protein domain, responsible for catalytic activity. The structure of this kinase domain is well-known from both c-Raf[27] and B-Raf.[28] It is highly similar to other Raf kinases and KSR proteins, and distinctly similar to some other MAP3 kinases, such as the Mixed Lineage Kinase (MLK) family. Together they comprise the Tyrosine Kinase Like (TKL) group of protein kinases. Although some features unite their catalytic domains with protein tyrosine kinases, the activity of TKLs is restricted to the phosphorylation of serine and threonine residues within target proteins. The most important substrate of Raf kinases (apart from itself) are the MKK1 and MKK2 kinases, whose activity strictly depends on phosphorylation events performed by Rafs.
Evolutionary relationships
Human c-Raf is a member of a larger family of related protein kinases. Two further members - found in most vertebrates - belong to the same family: B-Raf and A-Raf. Apart from the different length of their non-conserved N- and C-terminal ends, they all share the same domain architecture, structure and regulation. In comparison to the relatively well-known c-Raf and B-Raf, there is very little known of the precise function of A-Raf, but it is also thought to be similar to the other two members of the family. All these genes are believed to be the product of full gene or genome duplications at the dawn of vertebrate evolution, from a single ancestral Raf gene. Most other animal organisms possess only a single Raf gene. It is called Phl or Draf in Drosophila[29] and Lin-45 in C. elegans.[30]

The family of Raf kinases (schematic architectures)
Multicellular animals also have a type of kinase closely related to Raf: this is the Kinase Suppressor of Ras (KSR). Vertebrates like mammals have two, paralogous KSR genes instead of one: KSR1 and KSR2. Their C-terminal kinase domain is very similar to Raf (originally called CA5 in KSR and CR3 in Raf), but the N-terminal regulatory region differs. Although they also have the flexible hinge (CA4 in KSR) and a C1 domain (CA3 in KSR) before it, KSRs entirely lack the Ras-binding domain. Instead, they have unique regulatory regions on their N-termini, originally termed CA1 ("conserved area 1") and CA2. For a long time, the structure of the CA1 domain was a mystery. However, in 2012, the structure of the CA1 region in KSR1 was solved: it turned out to be a divergent SAM (sterile alpha motif) domain, supplemented with coiled-coils (CC-SAM): this is supposed to aid KSRs in membrane binding.[31] KSRs, like Rafs, also have the twin 14-3-3 associating motifs (that depend on phosphorylation), but also possess novel MAPK-binding motifs on their hinge regions. With a typical sequence Phe-x-Phe-Pro (FxFP) these motifs are important for the feedback regulation of Raf kinases in the ERK1/2 pathway. According to our current knowledge, KSRs also participate in the same pathway as Raf, although they only play an auxiliary role. With a very poor intrinsic kinase activity, they were long thought to be inactive, until their catalytic activity was finally demonstrated in recent years.[32][33] But even then, they contribute only negligibly to MKK1 and MKK2 phosphorylation. The main role of KSR appears to be to provide a heterodimerization partner to Raf enzymes, greatly facilitating their activation by means of allostery. Similar phenomena were described for other MAP3 kinases. ASK2, for example, is a poor enzyme on its own, and it activity appears to be tied to ASK1/ASK2 heterodimerisation.[34]
Raf-like kinases are fully absent from fungi. But recent sequencing of other opisthokonts (e.g. Capsaspora owczarzaki) revealed the presence of genuine Raf kinases in unicellular eukaryotes. Therefore, it is possible that Raf proteins are an ancient heritage and ancestors of fungi secondarily lost Raf-dependent signaling. Fungal MAP kinase pathways that are homologous to the mammalian ERK1/2 pathway (Fus3 and Kss1 in yeast) are activated by MEKK-related kinases (e.g. Ste11 in yeast) instead of Raf enzymes.
Raf kinases found in retroviruses (such as murine v-Raf) are secondarily derived from the corresponding vertebrate genes of their hosts. These Raf genes encode severely truncated proteins, that lack the entire N-terminal autoinhibitory domain, and the 14-3-3 binding motifs. Such severe truncations are known to induce an uncontrolled activity of Raf kinases: that is just exactly what a virus may need for efficient reproduction.
Regulation of activity
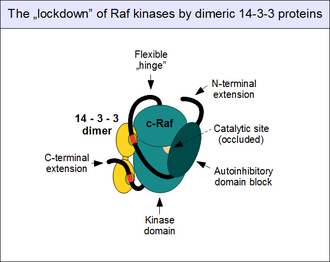
Artist's impression of the autoinhibited state of c-Raf, reinforced by the associated 14-3-3 protein dimers, bound to the phosphorylated twin motifs.[35][36]
As mentioned above, the regulation of c-Raf activity is complex. As a "gatekeeper" of the ERK1/2 pathway, it is kept in check by a multitude of inhibitory mechanisms, and normally cannot be activated in a single step. The most important regulatory mechanism involves the direct, physical association of the N-terminal autoinhibitory block to the kinase domain of c-Raf. It results in the occlusion of the catalytic site and full shutdown of kinase activity.[25] This "closed" state can only be relieved if the autoinhibitory domain of Raf engages a partner competing with its own kinase domain, most importantly GTP-bound Ras. Activated small G-proteins can thus break up the intramolacular interactions: this results in a conformational change ("opening") of c-Raf[37] necessary for kinase activation and substrate binding.
14-3-3 proteins also contribute to the autoinhibition. As 14-3-3 proteins are all known to form constitutive dimers, their assemblies have two binding sites.[38] Thus the dimer acts as a "molecular handcuff", locking their binding partners at a fixed distance and orientation. When the precisely positioned twin 14-3-3 binding motifs are engaged by a single 14-3-3 protein dimer (such as 14-3-3 zeta), they become locked into a conformation that promotes autoinhibition and does not allow the disengagement of the autoinhibitory and catalytic domains.[39] This "lockdown" of c-Raf (and other Rafs as well as KSRs) is controlled by motif phosphorylation. Unphosphorylated 14-3-3 associating motifs do not bind their partners: they need to get phosphorylated on conserved serines (Ser 259 and Ser 621) first, by other protein kinases. The most important kinase implicated in this event is TGF-beta activated kinase 1 (TAK1), and the enzymes dedicated for removal of these phosphates are the protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) complexes.[40][41]
Note that 14-3-3 binding of Raf enzymes is not necessarily inhibitory: once Raf is open and dimerizes, 14-3-3s can also bind in trans, bridging two kinases and "handcuffing" them together to reinforce the dimer, instead of keeping them away from each other.[42] Further modes of 14-3-3 interactions with c-Raf also exist, but their role is not well known.[43]
Dimerisation is another important mechanism for c-Raf activity regulation and required for Raf activation loop phosphorylation. Normally, only the "open" kinase domains participate in dimerisation. Unlike B-Raf, that readily forms homodimers with itself, c-Raf prefers heterodimerisation with either B-Raf or KSR1. Homodimers and heterodimers all behave similarly.[33] The B-Raf homodimer kinase domain structure clearly shows that the activation loops (that control the catalytic activity of all known protein kinases) are positioned in an active-like conformation in the dimer. This is due to an allosteric effect of the other molecule binding to the "back" side of the kinase; such dimers are symmetric and have two, partially active catalytic sites. At this stage, the activity of Raf kinases is low, and unstable.

The activation cycle of mammalian Raf proteins, exemplified by B-Raf (a greatly simplified overview, not showing all steps).[35][36]
To achieve full activity and stabilize the active state, the activation loop of c-Raf needs to be phosphorylated. The only kinases currently known to perform this act are the Raf family kinases themselves. But some other kinases, such as PAK1 can phosphorylate other residues near the kinase domain of c-Raf: the precise role of these auxiliary kinases is unknown. In the context of c-Raf, both c-Raf and KSR1 are needed for the "transphosphorylation" step. Due to the architecture of the dimers, this phosphorylation can only take place in trans (i.e. one dimer phosphorylates another, in a four-membered transitional complex).[44] By interacting with conserved Arg and Lys residues in the kinase domain, the phosphorylated activation loops shift conformation and become ordered, permanently locking the kinase domain into a fully active state until dephosphorylated. The phosphorylated activation loops also render the kinase insensitive to the presence of its autoinhibitory domain.[45] KSRs cannot undergo this last step as they miss any phosphorylatable residues in their activation loops. But once c-Raf is fully activated, there is no further need to do so: active Raf enzymes can now engage their substrates.[46] Like most protein kinases, c-Raf has multiple substrates. BAD (Bcl2-atagonist of cell death) is directly phosphorylated by c-Raf,[47] along with several types of adenylate cyclases,[48]myosin phosphatase (MYPT),[49]cardiac muscle troponin T (TnTc),[50] etc. The retinoblastoma protein (pRb) and Cdc25 phosphatase were also suggested as possible substrates.[51]
The most important targets of all Raf enzymes are MKK1(MEK1) and MKK2(MEK2). Although the structure of the enzyme-substrate complex c-Raf:MKK1 is unknown, it can be precisely modelled after the KSR2:MKK1 complex.[33] Here no actual catalysis takes place, but it is thought to be highly similar to the way Raf binds to its substrates. The main interaction interface is provided by the C-terminal lobes of both kinase domains; the large, disordered, proline-rich loop unique to MKK1 and MKK2 also plays an important role in its positioning to Raf (and KSR).[52] These MKKs become phosphorylated at at least two sites on their activation loops upon binding to Raf: this will activate them too. The targets of the kinase cascade are ERK1 and ERK2, that are selectively activated by MKK1 or MKK2. ERKs have numerous substrates in cells; they are also capable of translocating into the nucleus to activate nuclear transcription factors. Activated ERKs are pleiotropic effectors of cell physiology and play an important role in the control of gene expression involved in the cell division cycle, cell migration, inhibition of apoptosis, and cell differentiation.
Associated human diseases
Hereditary gain-of-function mutations of c-Raf are implicated in some rare, but severe syndromes. Most of these mutations involve single amino acid changes at one of the two 14-3-3 binding motifs.[53][54] Mutation of c-Raf is one of the possible causes of Noonan syndrome: affected individuals have congenital heart defects, short and dysmorphic stature and several other deformities. Similar mutations in c-Raf can also cause a related condition, termed LEOPARD syndrome (Lentigo, Electrocardiographic abnormalities, Ocular hypertelorism, Pulmonary stenosis, Abnormal genitalia, Retarded growth, Deafness), with a complex association of defects.
Role in cancer
Although c-Raf is very clearly capable of mutating into an oncogene in experimental settings, and even in a few human tumors,[55][56] its sister kinase B-Raf is the true major player in carcinogenesis in humans.[57]
B-Raf mutations
Approximately 20% of all examined human tumor samples display a mutated B-Raf gene.[58] The overwhelming majority of these mutations involve the exchange of a single amino acid: Val 600 into Glu,and this aberrant gene product (BRAF-V600E) can be visualized by immunohistochemistry for clinical molecular diagnostics[59][60] The aberration can mimic the activation loop phosphorylation and - by jumping all control steps at normal activation - immediately render the kinase domain fully active.[61] Since B-Raf can also activate itself by homodimerisation and c-Raf by heterodimerisation, this mutation has a catastrophic effect by turning the ERK1/2 pathway constitutively active, and driving an uncontrolled process of cell division.[62]
As a therapeutic target
Due to the importance of both Ras and B-Raf mutations in tumorigenesis, several Raf inhibitors were developed to combat cancer, especially against B-Raf exhibiting the V600E mutation. Sorafenib was the first clinically useful agent, that provides a pharmacological alternative to treat previously largely untreatable malignacies, such as renal cell carcinoma and melanoma.[63] Several other molecules followed up, such as Vemurafenib, Regorafenib, Dabrafenib, etc.
Unfortunately, ATP-competitive B-Raf inhibitors may have an undesired effect in K-Ras-dependent cancers: They are simply too selective for B-Raf. While they perfectly well inhibit B-Raf activity in case a mutant B-Raf is the primary culprit, they also promote homo- and heterodimerisation of B-Raf, with itself and c-Raf. This will actually enhance c-Raf activation instead of inhibiting it in case there is no mutation in any Raf genes, but their common upstream activator K-Ras protein is the one mutated.[27] This "paradoxical" c-Raf activation necessitates the need to screen for B-Raf mutations in patients (by genetic diagnostics) before starting a B-Raf-inhibitor therapy.[64]
List of interacting proteins
C-Raf has been shown to interact with:
AKT1,[65]
ASK1,[66]
BAG1,[67]
BRAF,[68]
Bcl-2,[69]
CDC25A,[70][71]
CFLAR,[72]
FYN,[73]
GRB10,[74][75]
HRAS,[76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92]
HSP90AA1,[93][94]
KRAS,[81][82]
MAP2K1,[95]
MAP3K1,[96]
MAPK7,[97]
MAPK8IP3,[98][99]
PAK1,[100]
PEBP1,[95]
PHB,[101]
PRKCZ,[102]
RAP1A,[17][86][103][104]
RHEB,[105][106][107]
RRAS2[81][108]
RB1,[101][109]
RBL2,[109]
SHOC2,[81]
STUB1,[93]
Src,[73]
TSC22D3,[110]
YWHAB,[80][102][111][112][113][114]
YWHAE,[113][114]
YWHAG,[102][115][116]
YWHAH,[102][113][117]
YWHAQ,[95][102][115][118] and
YWHAZ.[102][119][120][121][122]
See also
- Raf kinases
- A-Raf kinase
- B-Raf kinase
- KSR1 protein
- KSR2 protein
References
^ abc GRCm38: Ensembl release 89: ENSMUSG00000000441 - Ensembl, May 2017
^ "Human PubMed Reference:"..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ "Mouse PubMed Reference:".
^ Li P, Wood K, Mamon H, Haser W, Roberts T (February 1991). "Raf-1: a kinase currently without a cause but not lacking in effects". Cell. 64 (3): 479–82. doi:10.1016/0092-8674(91)90228-Q. PMID 1846778.
^ ab Rapp UR, Goldsborough MD, Mark GE, Bonner TI, Groffen J, Reynolds FH, Stephenson JR (July 1983). "Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus". Proc. Natl. Acad. Sci. U.S.A. 80 (14): 4218–22. Bibcode:1983PNAS...80.4218R. doi:10.1073/pnas.80.14.4218. PMC 384008. PMID 6308607.
^ Bonner T, O'Brien SJ, Nash WG, Rapp UR, Morton CC, Leder P (January 1984). "The human homologs of the raf (mil) oncogene are located on human chromosomes 3 and 4". Science. 223 (4631): 71–4. Bibcode:1984Sci...223...71B. doi:10.1126/science.6691137. PMID 6691137.
^ "Entrez Gene: RAF1 v-raf-1 murine leukemia viral oncogene homolog 1".
^ Sutrave P, Bonner TI, Rapp UR, Jansen HW, Patschinsky T, Bister K (1984). "Nucleotide sequence of avian retroviral oncogene v-mil: homologue of murine retroviral oncogene v-raf". Nature. 309 (5963): 85–8. Bibcode:1984Natur.309...85S. doi:10.1038/309085a0. PMID 6325930.
^ Moelling K, Heimann B, Beimling P, Rapp UR, Sander T (1984). "Serine- and threonine-specific protein kinase activities of purified gag-mil and gag-raf proteins". Nature. 312 (5994): 558–61. Bibcode:1984Natur.312..558M. doi:10.1038/312558a0. PMID 6438534.
^ Kolch W, Heidecker G, Lloyd P, Rapp UR (January 1991). "Raf-1 protein kinase is required for growth of induced NIH/3T3 cells". Nature. 349 (6308): 426–8. Bibcode:1991Natur.349..426K. doi:10.1038/349426a0. PMID 1992343.
^ Mark GE, Rapp UR (April 1984). "Primary structure of v-raf: relatedness to the src family of oncogenes". Science. 224 (4646): 285–9. Bibcode:1984Sci...224..285M. doi:10.1126/science.6324342. PMID 6324342.
^ Kyriakis JM, App H, Zhang XF, Banerjee P, Brautigan DL, Rapp UR, Avruch J (July 1992). "Raf-1 activates MAP kinase-kinase". Nature. 358 (6385): 417–21. Bibcode:1992Natur.358..417K. doi:10.1038/358417a0. PMID 1322500.
^ Shimizu K, Nakatsu Y, Nomoto S, Sekiguchi M (1986). "Structure of the activated c-raf-1 gene from human stomach cancer". Int. Symp. Princess Takamatsu Cancer Res. Fund. 17: 85–91. PMID 2843497.
^ Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA (June 2002). "Mutations of the BRAF gene in human cancer". Nature. 417 (6892): 949–54. doi:10.1038/nature00766. PMID 12068308.
^ Sridhar SS, Hedley D, Siu LL (April 2005). "Raf kinase as a target for anticancer therapeutics". Mol. Cancer Ther. 4 (4): 677–85. doi:10.1158/1535-7163.MCT-04-0297. PMID 15827342.
^ Dozier C, Ansieau S, Ferreira E, Coll J, Stehelin D (August 1991). "An alternatively spliced c-mil/raf mRNA is predominantly expressed in chicken muscular tissues and conserved among vertebrate species". Oncogene. 6 (8): 1307–11. PMID 1886707.
^ ab Nassar N, Horn G, Herrmann C, Scherer A, McCormick F, Wittinghofer A (June 1995). "The 2.2 A crystal structure of the Ras-binding domain of the serine/threonine kinase c-Raf1 in complex with Rap1A and a GTP analogue". Nature. 375 (6532): 554–60. Bibcode:1995Natur.375..554N. doi:10.1038/375554a0. PMID 7791872.
^ Emerson SD, Madison VS, Palermo RE, Waugh DS, Scheffler JE, Tsao KL, Kiefer SE, Liu SP, Fry DC (May 1995). "Solution structure of the Ras-binding domain of c-Raf-1 and identification of its Ras interaction surface". Biochemistry. 34 (21): 6911–8. doi:10.1021/bi00021a001. PMID 7766599.
^ Moodie SA, Willumsen BM, Weber MJ, Wolfman A (June 1993). "Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase". Science. 260 (5114): 1658–61. Bibcode:1993Sci...260.1658M. doi:10.1126/science.8503013. PMID 8503013.
^ Mott HR, Carpenter JW, Zhong S, Ghosh S, Bell RM, Campbell SL (August 1996). "The solution structure of the Raf-1 cysteine-rich domain: a novel ras and phospholipid binding site". Proc. Natl. Acad. Sci. U.S.A. 93 (16): 8312–7. Bibcode:1996PNAS...93.8312M. doi:10.1073/pnas.93.16.8312. PMC 38667. PMID 8710867.
^ ab Daub M, Jöckel J, Quack T, Weber CK, Schmitz F, Rapp UR, Wittinghofer A, Block C (November 1998). "The RafC1 cysteine-rich domain contains multiple distinct regulatory epitopes which control Ras-dependent Raf activation". Mol. Cell. Biol. 18 (11): 6698–710. PMC 109253. PMID 9774683.
^ ab Yin X, Zafrullah M, Lee H, Haimovitz-Friedman A, Fuks Z, Kolesnick R (2009). "A ceramide-binding C1 domain mediates kinase suppressor of ras membrane translocation". Cell. Physiol. Biochem. 24 (3–4): 219–30. doi:10.1159/000233248. PMC 2978518. PMID 19710537.
^ Kraft CA, Garrido JL, Fluharty E, Leiva-Vega L, Romero G (December 2008). "Role of phosphatidic acid in the coupling of the ERK cascade". J. Biol. Chem. 283 (52): 36636–45. doi:10.1074/jbc.M804633200. PMC 2606017. PMID 18952605.
^ Brtva TR, Drugan JK, Ghosh S, Terrell RS, Campbell-Burk S, Bell RM, Der CJ (April 1995). "Two distinct Raf domains mediate interaction with Ras". J. Biol. Chem. 270 (17): 9809–12. doi:10.1074/jbc.270.17.9809. PMID 7730360.
^ ab Cutler RE, Stephens RM, Saracino MR, Morrison DK (August 1998). "Autoregulation of the Raf-1 serine/threonine kinase". Proc. Natl. Acad. Sci. U.S.A. 95 (16): 9214–9. Bibcode:1998PNAS...95.9214C. doi:10.1073/pnas.95.16.9214. PMC 21318. PMID 9689060.
^ Hmitou I, Druillennec S, Valluet A, Peyssonnaux C, Eychène A (January 2007). "Differential regulation of B-raf isoforms by phosphorylation and autoinhibitory mechanisms". Mol. Cell. Biol. 27 (1): 31–43. doi:10.1128/MCB.01265-06. PMC 1800654. PMID 17074813.
^ ab Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor SL, Vigers G, Morales T, Aliagas I, Liu B, Sideris S, Hoeflich KP, Jaiswal BS, Seshagiri S, Koeppen H, Belvin M, Friedman LS, Malek S (March 2010). "RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth". Nature. 464 (7287): 431–5. Bibcode:2010Natur.464..431H. doi:10.1038/nature08833. PMID 20130576.
^ Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R (March 2004). "Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF". Cell. 116 (6): 855–67. doi:10.1016/S0092-8674(04)00215-6. PMID 15035987.
^ Mark GE, MacIntyre RJ, Digan ME, Ambrosio L, Perrimon N (June 1987). "Drosophila melanogaster homologs of the raf oncogene". Mol. Cell. Biol. 7 (6): 2134–40. PMC 365335. PMID 3037346.
^ Chong H, Vikis HG, Guan KL (May 2003). "Mechanisms of regulating the Raf kinase family". Cell. Signal. 15 (5): 463–9. doi:10.1016/S0898-6568(02)00139-0. PMID 12639709.
^ Koveal D, Schuh-Nuhfer N, Ritt D, Page R, Morrison DK, Peti W (December 2012). "A CC-SAM, for coiled coil-sterile α motif, domain targets the scaffold KSR-1 to specific sites in the plasma membrane". Sci Signal. 5 (255): ra94. doi:10.1126/scisignal.2003289. PMC 3740349. PMID 23250398.
^ Hu J, Yu H, Kornev AP, Zhao J, Filbert EL, Taylor SS, Shaw AS (April 2011). "Mutation that blocks ATP binding creates a pseudokinase stabilizing the scaffolding function of kinase suppressor of Ras, CRAF and BRAF". Proc. Natl. Acad. Sci. U.S.A. 108 (15): 6067–72. Bibcode:2011PNAS..108.6067H. doi:10.1073/pnas.1102554108. PMC 3076888. PMID 21441104.
^ abc Brennan DF, Dar AC, Hertz NT, Chao WC, Burlingame AL, Shokat KM, Barford D (April 2011). "A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK". Nature. 472 (7343): 366–9. Bibcode:2011Natur.472..366B. doi:10.1038/nature09860. PMID 21441910.
^ Ortner E, Moelling K (October 2007). "Heteromeric complex formation of ASK2 and ASK1 regulates stress-induced signaling". Biochem. Biophys. Res. Commun. 362 (2): 454–9. doi:10.1016/j.bbrc.2007.08.006. PMID 17714688.
^ ab Matallanas D, Birtwistle M, Romano D, Zebisch A, Rauch J, von Kriegsheim A, Kolch W (2011). "Raf family kinases: old dogs have learned new tricks". Genes Cancer. 2 (3): 232–60. doi:10.1177/1947601911407323. PMC 3128629. PMID 21779496.
^ ab Alexa A, Varga J, Reményi A (2010). "Scaffolds are 'active' regulators of signaling modules". FEBS J. 277 (21): 4376–82. doi:10.1111/j.1742-4658.2010.07867.x. PMID 20883493.
^ Terai K, Matsuda M (March 2005). "Ras binding opens c-Raf to expose the docking site for mitogen-activated protein kinase kinase". EMBO Rep. 6 (3): 251–5. doi:10.1038/sj.embor.7400349. PMC 1299259. PMID 15711535.
^ Liu D, Bienkowska J, Petosa C, Collier RJ, Fu H, Liddington R (July 1995). "Crystal structure of the zeta isoform of the 14-3-3 protein". Nature. 376 (6536): 191–4. Bibcode:1995Natur.376..191L. doi:10.1038/376191a0. PMID 7603574.
^ Fischer A, Baljuls A, Reinders J, Nekhoroshkova E, Sibilski C, Metz R, Albert S, Rajalingam K, Hekman M, Rapp UR (January 2009). "Regulation of RAF activity by 14-3-3 proteins: RAF kinases associate functionally with both homo- and heterodimeric forms of 14-3-3 proteins". J. Biol. Chem. 284 (5): 3183–94. doi:10.1074/jbc.M804795200. PMID 19049963.
^ Rodriguez-Viciana P, Oses-Prieto J, Burlingame A, Fried M, McCormick F (April 2006). "A phosphatase holoenzyme comprised of Shoc2/Sur8 and the catalytic subunit of PP1 functions as an M-Ras effector to modulate Raf activity". Mol. Cell. 22 (2): 217–30. doi:10.1016/j.molcel.2006.03.027. PMID 16630891.
^ Jaumot M, Hancock JF (July 2001). "Protein phosphatases 1 and 2A promote Raf-1 activation by regulating 14-3-3 interactions". Oncogene. 20 (30): 3949–58. doi:10.1038/sj.onc.1204526. PMID 11494123.
^ Tzivion G, Luo Z, Avruch J (July 1998). "A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity". Nature. 394 (6688): 88–92. Bibcode:1998Natur.394...88T. doi:10.1038/27938. PMID 9665134.
^ Molzan M, Ottmann C (November 2012). "Synergistic binding of the phosphorylated S233- and S259-binding sites of C-RAF to one 14-3-3ζ dimer". J. Mol. Biol. 423 (4): 486–95. doi:10.1016/j.jmb.2012.08.009. PMID 22922483.
^ McKay MM, Freeman AK, Morrison DK (2011). "Complexity in KSR function revealed by Raf inhibitor and KSR structure studies". Small GTPases. 2 (5): 276–281. doi:10.4161/sgtp.2.5.17740. PMC 3265819. PMID 22292131.
^ Chong H, Guan KL (September 2003). "Regulation of Raf through phosphorylation and N terminus-C terminus interaction". J. Biol. Chem. 278 (38): 36269–76. doi:10.1074/jbc.M212803200. PMID 12865432.
^ Shi F, Lemmon MA (May 2011). "Biochemistry. KSR plays CRAF-ty". Science. 332 (6033): 1043–4. Bibcode:2011Sci...332.1043S. doi:10.1126/science.1208063. PMID 21617065.
^ Ye DZ, Jin S, Zhuo Y, Field J (2011). Bauer JA, ed. "p21-Activated kinase 1 (Pak1) phosphorylates BAD directly at serine 111 in vitro and indirectly through Raf-1 at serine 112". PLoS ONE. 6 (11): e27637. Bibcode:2011PLoSO...627637Y. doi:10.1371/journal.pone.0027637. PMC 3214075. PMID 22096607.
^ Ding Q, Gros R, Gray ID, Taussig R, Ferguson SS, Feldman RD (October 2004). "Raf kinase activation of adenylyl cyclases: isoform-selective regulation". Mol. Pharmacol. 66 (4): 921–8. doi:10.1124/mol.66.4.921. PMID 15385642.
^ Broustas CG, Grammatikakis N, Eto M, Dent P, Brautigan DL, Kasid U (January 2002). "Phosphorylation of the myosin-binding subunit of myosin phosphatase by Raf-1 and inhibition of phosphatase activity". J. Biol. Chem. 277 (4): 3053–9. doi:10.1074/jbc.M106343200. PMID 11719507.
^ Pfleiderer P, Sumandea MP, Rybin VO, Wang C, Steinberg SF (2009). "Raf-1: a novel cardiac troponin T kinase". J. Muscle Res. Cell. Motil. 30 (1–2): 67–72. doi:10.1007/s10974-009-9176-y. PMC 2893395. PMID 19381846.
^ Hindley A, Kolch W (April 2002). "Extracellular signal regulated kinase (ERK)/mitogen activated protein kinase (MAPK)-independent functions of Raf kinases". J. Cell Sci. 115 (Pt 8): 1575–81. PMID 11950876.
^ Catling AD, Schaeffer HJ, Reuter CW, Reddy GR, Weber MJ (October 1995). "A proline-rich sequence unique to MEK1 and MEK2 is required for raf binding and regulates MEK function". Mol. Cell. Biol. 15 (10): 5214–25. PMC 230769. PMID 7565670.
^ Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, Pogna EA, Schackwitz W, Ustaszewska A, Landstrom A, Bos JM, Ommen SR, Esposito G, Lepri F, Faul C, Mundel P, López Siguero JP, Tenconi R, Selicorni A, Rossi C, Mazzanti L, Torrente I, Marino B, Digilio MC, Zampino G, Ackerman MJ, Dallapiccola B, Tartaglia M, Gelb BD (August 2007). "Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy". Nat. Genet. 39 (8): 1007–12. doi:10.1038/ng2073. PMID 17603483.
^ Molzan M, Schumacher B, Ottmann C, Baljuls A, Polzien L, Weyand M, Thiel P, Rose R, Rose M, Kuhenne P, Kaiser M, Rapp UR, Kuhlmann J, Ottmann C (October 2010). "Impaired binding of 14-3-3 to C-RAF in Noonan syndrome suggests new approaches in diseases with increased Ras signaling". Mol. Cell. Biol. 30 (19): 4698–711. doi:10.1128/MCB.01636-09. PMC 2950525. PMID 20679480.
^ Storm SM, Rapp UR (April 1993). "Oncogene activation: c-raf-1 gene mutations in experimental and naturally occurring tumors". Toxicol. Lett. 67 (1–3): 201–10. doi:10.1016/0378-4274(93)90056-4. PMID 8451761.
^ Zebisch A, Staber PB, Delavar A, Bodner C, Hiden K, Fischereder K, Janakiraman M, Linkesch W, Auner HW, Emberger W, Windpassinger C, Schimek MG, Hoefler G, Troppmair J, Sill H (April 2006). "Two transforming C-RAF germ-line mutations identified in patients with therapy-related acute myeloid leukemia". Cancer Res. 66 (7): 3401–8. doi:10.1158/0008-5472.CAN-05-0115. PMID 16585161.
^ Emuss V, Garnett M, Mason C, Marais R (November 2005). "Mutations of C-RAF are rare in human cancer because C-RAF has a low basal kinase activity compared with B-RAF". Cancer Res. 65 (21): 9719–26. doi:10.1158/0008-5472.CAN-05-1683. PMID 16266992.
^ Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, Teague JW, Campbell PJ, Stratton MR, Futreal PA (January 2011). "COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer". Nucleic Acids Res. 39 (Database issue): D945–50. doi:10.1093/nar/gkq929. PMC 3013785. PMID 20952405.
^ Capper D, Berghoff AS, Magerle M, Ilhan A, Wöhrer A, Hackl M, Pichler J, Pusch S, Meyer J, Habel A, Petzelbauer P, Birner P, von Deimling A, Preusser M (2012). "Immunohistochemical testing of BRAF V600E status in 1,120 tumor tissue samples of patients with brain metastases". Acta Neuropathol. 123 (2): 223–33. doi:10.1007/s00401-011-0887-y. PMID 22012135.
^ Capper D, Preusser M, Habel A, Sahm F, Ackermann U, Schindler G, Pusch S, Mechtersheimer G, Zentgraf H, von Deimling A (2011). "Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody". Acta Neuropathol. 122 (1): 11–9. doi:10.1007/s00401-011-0841-z. PMID 21638088.
^ Tran NH, Wu X, Frost JA (April 2005). "B-Raf and Raf-1 are regulated by distinct autoregulatory mechanisms". J. Biol. Chem. 280 (16): 16244–53. doi:10.1074/jbc.M501185200. PMID 15710605.
^ Garnett MJ, Rana S, Paterson H, Barford D, Marais R (December 2005). "Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization". Mol. Cell. 20 (6): 963–9. doi:10.1016/j.molcel.2005.10.022. PMID 16364920.
^ Maurer G, Tarkowski B, Baccarini M (August 2011). "Raf kinases in cancer-roles and therapeutic opportunities". Oncogene. 30 (32): 3477–88. doi:10.1038/onc.2011.160. PMID 21577205.
^ Kim DH, Sim T (March 2012). "Novel small molecule Raf kinase inhibitors for targeted cancer therapeutics". Arch. Pharm. Res. 35 (4): 605–15. doi:10.1007/s12272-012-0403-5. PMID 22553052.
^ Zimmermann S, Moelling K (November 1999). "Phosphorylation and regulation of Raf by Akt (protein kinase B)". Science. 286 (5445): 1741–4. doi:10.1126/science.286.5445.1741. PMID 10576742.
^ Chen J, Fujii K, Zhang L, Roberts T, Fu H (July 2001). "Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism". Proc. Natl. Acad. Sci. U.S.A. 98 (14): 7783–8. Bibcode:2001PNAS...98.7783C. doi:10.1073/pnas.141224398. PMC 35419. PMID 11427728.
^ Wang HG, Takayama S, Rapp UR, Reed JC (July 1996). "Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1". Proc. Natl. Acad. Sci. U.S.A. 93 (14): 7063–8. Bibcode:1996PNAS...93.7063W. doi:10.1073/pnas.93.14.7063. PMC 38936. PMID 8692945.
^ Weber CK, Slupsky JR, Kalmes HA, Rapp UR (May 2001). "Active Ras induces heterodimerization of cRaf and BRaf". Cancer Res. 61 (9): 3595–8. PMID 11325826.
^ Wang HG, Rapp UR, Reed JC (November 1996). "Bcl-2 targets the protein kinase Raf-1 to mitochondria". Cell. 87 (4): 629–38. doi:10.1016/s0092-8674(00)81383-5. PMID 8929532.
^ Galaktionov K, Jessus C, Beach D (May 1995). "Raf1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation". Genes Dev. 9 (9): 1046–58. doi:10.1101/gad.9.9.1046. PMID 7744247.
^ Huang TS, Shu CH, Yang WK, Whang-Peng J (July 1997). "Activation of CDC 25 phosphatase and CDC 2 kinase involved in GL331-induced apoptosis". Cancer Res. 57 (14): 2974–8. PMID 9230211.
^ Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M, Tschopp J (June 2000). "The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways". Curr. Biol. 10 (11): 640–8. doi:10.1016/s0960-9822(00)00512-1. PMID 10837247.
^ ab Cleghon V, Morrison DK (July 1994). "Raf-1 interacts with Fyn and Src in a non-phosphotyrosine-dependent manner". J. Biol. Chem. 269 (26): 17749–55. PMID 7517401.
^ Nantel A, Huber M, Thomas DY (December 1999). "Localization of endogenous Grb10 to the mitochondria and its interaction with the mitochondrial-associated Raf-1 pool". J. Biol. Chem. 274 (50): 35719–24. doi:10.1074/jbc.274.50.35719. PMID 10585452.
^ Nantel A, Mohammad-Ali K, Sherk J, Posner BI, Thomas DY (April 1998). "Interaction of the Grb10 adapter protein with the Raf1 and MEK1 kinases". J. Biol. Chem. 273 (17): 10475–84. doi:10.1074/jbc.273.17.10475. PMID 9553107.
^ Stang S, Bottorff D, Stone JC (June 1997). "Interaction of activated Ras with Raf-1 alone may be sufficient for transformation of rat2 cells". Mol. Cell. Biol. 17 (6): 3047–55. PMC 232157. PMID 9154803.
^ Germani A, Prabel A, Mourah S, Podgorniak MP, Di Carlo A, Ehrlich R, Gisselbrecht S, Varin-Blank N, Calvo F, Bruzzoni-Giovanelli H (December 2003). "SIAH-1 interacts with CtIP and promotes its degradation by the proteasome pathway". Oncogene. 22 (55): 8845–51. doi:10.1038/sj.onc.1206994. PMID 14654780.
^ Mitin NY, Ramocki MB, Zullo AJ, Der CJ, Konieczny SF, Taparowsky EJ (May 2004). "Identification and characterization of rain, a novel Ras-interacting protein with a unique subcellular localization". J. Biol. Chem. 279 (21): 22353–61. doi:10.1074/jbc.M312867200. PMID 15031288.
^ Vargiu P, De Abajo R, Garcia-Ranea JA, Valencia A, Santisteban P, Crespo P, Bernal J (January 2004). "The small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptors". Oncogene. 23 (2): 559–68. doi:10.1038/sj.onc.1207161. PMID 14724584.
^ ab Yuryev A, Wennogle LP (February 2003). "Novel raf kinase protein-protein interactions found by an exhaustive yeast two-hybrid analysis". Genomics. 81 (2): 112–25. doi:10.1016/s0888-7543(02)00008-3. PMID 12620389.
^ abcd Li W, Han M, Guan KL (April 2000). "The leucine-rich repeat protein SUR-8 enhances MAP kinase activation and forms a complex with Ras and Raf". Genes Dev. 14 (8): 895–900. PMC 316541. PMID 10783161.
^ ab Kiyono M, Kato J, Kataoka T, Kaziro Y, Satoh T (September 2000). "Stimulation of Ras guanine nucleotide exchange activity of Ras-GRF1/CDC25(Mm) upon tyrosine phosphorylation by the Cdc42-regulated kinase ACK1". J. Biol. Chem. 275 (38): 29788–93. doi:10.1074/jbc.M001378200. PMID 10882715.
^ Janoueix-Lerosey I, Pasheva E, de Tand MF, Tavitian A, de Gunzburg J (March 1998). "Identification of a specific effector of the small GTP-binding protein Rap2". Eur. J. Biochem. 252 (2): 290–8. doi:10.1046/j.1432-1327.1998.2520290.x. PMID 9523700.
^ Boettner B, Govek EE, Cross J, Van Aelst L (August 2000). "The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin". Proc. Natl. Acad. Sci. U.S.A. 97 (16): 9064–9. Bibcode:2000PNAS...97.9064B. doi:10.1073/pnas.97.16.9064. PMC 16822. PMID 10922060.
^ Karbowniczek M, Robertson GP, Henske EP (September 2006). "Rheb inhibits C-raf activity and B-raf/C-raf heterodimerization". J. Biol. Chem. 281 (35): 25447–56. doi:10.1074/jbc.M605273200. PMID 16803888.
^ ab Han L, Colicelli J (March 1995). "A human protein selected for interference with Ras function interacts directly with Ras and competes with Raf1". Mol. Cell. Biol. 15 (3): 1318–23. doi:10.1128/mcb.15.3.1318. PMC 230355. PMID 7862125.
^ Jelinek T, Catling AD, Reuter CW, Moodie SA, Wolfman A, Weber MJ (December 1994). "RAS and RAF-1 form a signalling complex with MEK-1 but not MEK-2". Mol. Cell. Biol. 14 (12): 8212–8. PMC 359360. PMID 7969158.
^ Romero F, Martínez-A C, Camonis J, Rebollo A (June 1999). "Aiolos transcription factor controls cell death in T cells by regulating Bcl-2 expression and its cellular localization". EMBO J. 18 (12): 3419–30. doi:10.1093/emboj/18.12.3419. PMC 1171421. PMID 10369681.
^ Morcos P, Thapar N, Tusneem N, Stacey D, Tamanoi F (May 1996). "Identification of neurofibromin mutants that exhibit allele specificity or increased Ras affinity resulting in suppression of activated ras alleles". Mol. Cell. Biol. 16 (5): 2496–503. PMC 231238. PMID 8628317.
^ Hu CD, Kariya K, Tamada M, Akasaka K, Shirouzu M, Yokoyama S, Kataoka T (December 1995). "Cysteine-rich region of Raf-1 interacts with activator domain of post-translationally modified Ha-Ras". J. Biol. Chem. 270 (51): 30274–7. doi:10.1074/jbc.270.51.30274. PMID 8530446.
^ Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J (May 1997). "Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras". Cell. 89 (3): 457–67. doi:10.1016/s0092-8674(00)80226-3. PMID 9150145.
^ Huang YZ, Zang M, Xiong WC, Luo Z, Mei L (January 2003). "Erbin suppresses the MAP kinase pathway". J. Biol. Chem. 278 (2): 1108–14. doi:10.1074/jbc.M205413200. PMID 12379659.
^ ab Dogan T, Harms GS, Hekman M, Karreman C, Oberoi TK, Alnemri ES, Rapp UR, Rajalingam K (December 2008). "X-linked and cellular IAPs modulate the stability of C-RAF kinase and cell motility". Nat. Cell Biol. 10 (12): 1447–55. doi:10.1038/ncb1804. PMID 19011619.
^ Stancato LF, Chow YH, Hutchison KA, Perdew GH, Jove R, Pratt WB (October 1993). "Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell-free system". J. Biol. Chem. 268 (29): 21711–6. PMID 8408024.
^ abc Yeung K, Janosch P, McFerran B, Rose DW, Mischak H, Sedivy JM, Kolch W (May 2000). "Mechanism of suppression of the Raf/MEK/extracellular signal-regulated kinase pathway by the raf kinase inhibitor protein". Mol. Cell. Biol. 20 (9): 3079–85. doi:10.1128/mcb.20.9.3079-3085.2000. PMC 85596. PMID 10757792.
^ Karandikar M, Xu S, Cobb MH (December 2000). "MEKK1 binds raf-1 and the ERK2 cascade components". J. Biol. Chem. 275 (51): 40120–7. doi:10.1074/jbc.M005926200. PMID 10969079.
^ English JM, Pearson G, Hockenberry T, Shivakumar L, White MA, Cobb MH (October 1999). "Contribution of the ERK5/MEK5 pathway to Ras/Raf signaling and growth control". J. Biol. Chem. 274 (44): 31588–92. doi:10.1074/jbc.274.44.31588. PMID 10531364.
^ Kuboki Y, Ito M, Takamatsu N, Yamamoto KI, Shiba T, Yoshioka K (December 2000). "A scaffold protein in the c-Jun NH2-terminal kinase signaling pathways suppresses the extracellular signal-regulated kinase signaling pathways". J. Biol. Chem. 275 (51): 39815–8. doi:10.1074/jbc.C000403200. PMID 11044439.
^ Ito M, Yoshioka K, Akechi M, Yamashita S, Takamatsu N, Sugiyama K, Hibi M, Nakabeppu Y, Shiba T, Yamamoto KI (November 1999). "JSAP1, a novel jun N-terminal protein kinase (JNK)-binding protein that functions as a Scaffold factor in the JNK signaling pathway". Mol. Cell. Biol. 19 (11): 7539–48. doi:10.1128/mcb.19.11.7539. PMC 84763. PMID 10523642.
^ Zang M, Hayne C, Luo Z (February 2002). "Interaction between active Pak1 and Raf-1 is necessary for phosphorylation and activation of Raf-1". J. Biol. Chem. 277 (6): 4395–405. doi:10.1074/jbc.M110000200. PMID 11733498.
^ ab Wang S, Nath N, Fusaro G, Chellappan S (November 1999). "Rb and prohibitin target distinct regions of E2F1 for repression and respond to different upstream signals". Mol. Cell. Biol. 19 (11): 7447–60. doi:10.1128/mcb.19.11.7447. PMC 84738. PMID 10523633.
^ abcdef Van Der Hoeven PC, Van Der Wal JC, Ruurs P, Van Dijk MC, Van Blitterswijk J (January 2000). "14-3-3 isotypes facilitate coupling of protein kinase C-zeta to Raf-1: negative regulation by 14-3-3 phosphorylation". Biochem. J. 345 (2): 297–306. doi:10.1042/0264-6021:3450297. PMC 1220759. PMID 10620507.
^ Hu CD, Kariya K, Okada T, Qi X, Song C, Kataoka T (January 1999). "Effect of phosphorylation on activities of Rap1A to interact with Raf-1 and to suppress Ras-dependent Raf-1 activation". J. Biol. Chem. 274 (1): 48–51. doi:10.1074/jbc.274.1.48. PMID 9867809.
^ Okada T, Hu CD, Jin TG, Kariya K, Yamawaki-Kataoka Y, Kataoka T (September 1999). "The strength of interaction at the Raf cysteine-rich domain is a critical determinant of response of Raf to Ras family small GTPases". Mol. Cell. Biol. 19 (9): 6057–64. doi:10.1128/mcb.19.9.6057. PMC 84512. PMID 10454553.
^ Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J (April 2005). "Rheb binds and regulates the mTOR kinase". Curr. Biol. 15 (8): 702–13. doi:10.1016/j.cub.2005.02.053. PMID 15854902.
^ Karbowniczek M, Cash T, Cheung M, Robertson GP, Astrinidis A, Henske EP (July 2004). "Regulation of B-Raf kinase activity by tuberin and Rheb is mammalian target of rapamycin (mTOR)-independent". J. Biol. Chem. 279 (29): 29930–7. doi:10.1074/jbc.M402591200. PMID 15150271.
^ Yee WM, Worley PF (February 1997). "Rheb interacts with Raf-1 kinase and may function to integrate growth factor- and protein kinase A-dependent signals". Mol. Cell. Biol. 17 (2): 921–33. doi:10.1128/mcb.17.2.921. PMC 231818. PMID 9001246.
^ Movilla N, Crespo P, Bustelo XR (October 1999). "Signal transduction elements of TC21, an oncogenic member of the R-Ras subfamily of GTP-binding proteins". Oncogene. 18 (43): 5860–9. doi:10.1038/sj.onc.1202968. PMID 10557073.
^ ab Wang S, Ghosh RN, Chellappan SP (December 1998). "Raf-1 physically interacts with Rb and regulates its function: a link between mitogenic signaling and cell cycle regulation". Mol. Cell. Biol. 18 (12): 7487–98. doi:10.1128/mcb.18.12.7487. PMC 109329. PMID 9819434.
^ Ayroldi E, Zollo O, Macchiarulo A, Di Marco B, Marchetti C, Riccardi C (November 2002). "Glucocorticoid-induced leucine zipper inhibits the Raf-extracellular signal-regulated kinase pathway by binding to Raf-1". Mol. Cell. Biol. 22 (22): 7929–41. doi:10.1128/mcb.22.22.7929-7941.2002. PMC 134721. PMID 12391160.
^ Truong AB, Masters SC, Yang H, Fu H (November 2002). "Role of the 14-3-3 C-terminal loop in ligand interaction". Proteins. 49 (3): 321–5. doi:10.1002/prot.10210. PMID 12360521.
^ Yuryev A, Ono M, Goff SA, Macaluso F, Wennogle LP (July 2000). "Isoform-specific localization of A-RAF in mitochondria". Mol. Cell. Biol. 20 (13): 4870–8. doi:10.1128/mcb.20.13.4870-4878.2000. PMC 85938. PMID 10848612.
^ abc Vincenz C, Dixit VM (August 1996). "14-3-3 proteins associate with A20 in an isoform-specific manner and function both as chaperone and adapter molecules". J. Biol. Chem. 271 (33): 20029–34. doi:10.1074/jbc.271.33.20029. PMID 8702721.
^ ab Conklin DS, Galaktionov K, Beach D (August 1995). "14-3-3 proteins associate with cdc25 phosphatases". Proc. Natl. Acad. Sci. U.S.A. 92 (17): 7892–6. Bibcode:1995PNAS...92.7892C. doi:10.1073/pnas.92.17.7892. PMC 41252. PMID 7644510.
^ ab Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, McBroom-Cerajewski L, Robinson MD, O'Connor L, Li M, Taylor R, Dharsee M, Ho Y, Heilbut A, Moore L, Zhang S, Ornatsky O, Bukhman YV, Ethier M, Sheng Y, Vasilescu J, Abu-Farha M, Lambert JP, Duewel HS, Stewart II, Kuehl B, Hogue K, Colwill K, Gladwish K, Muskat B, Kinach R, Adams SL, Moran MF, Morin GB, Topaloglou T, Figeys D (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Mol. Syst. Biol. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
^ Autieri MV, Carbone CJ (July 1999). "14-3-3Gamma interacts with and is phosphorylated by multiple protein kinase C isoforms in PDGF-stimulated human vascular smooth muscle cells". DNA Cell Biol. 18 (7): 555–64. doi:10.1089/104454999315105. PMID 10433554.
^ Ichimura T, Wakamiya-Tsuruta A, Itagaki C, Taoka M, Hayano T, Natsume T, Isobe T (April 2002). "Phosphorylation-dependent interaction of kinesin light chain 2 and the 14-3-3 protein". Biochemistry. 41 (17): 5566–72. doi:10.1021/bi015946f. PMID 11969417.
^ Liu YC, Elly C, Yoshida H, Bonnefoy-Berard N, Altman A (June 1996). "Activation-modulated association of 14-3-3 proteins with Cbl in T cells". J. Biol. Chem. 271 (24): 14591–5. doi:10.1074/jbc.271.24.14591. PMID 8663231.
^ Clark GJ, Drugan JK, Rossman KL, Carpenter JW, Rogers-Graham K, Fu H, Der CJ, Campbell SL (August 1997). "14-3-3 zeta negatively regulates raf-1 activity by interactions with the Raf-1 cysteine-rich domain". J. Biol. Chem. 272 (34): 20990–3. doi:10.1074/jbc.272.34.20990. PMID 9261098.
^ Tzivion G, Luo ZJ, Avruch J (September 2000). "Calyculin A-induced vimentin phosphorylation sequesters 14-3-3 and displaces other 14-3-3 partners in vivo". J. Biol. Chem. 275 (38): 29772–8. doi:10.1074/jbc.M001207200. PMID 10887173.
^ Koyama S, Williams LT, Kikuchi A (July 1995). "Characterization of the interaction of Raf-1 with ras p21 or 14-3-3 protein in intact cells". FEBS Lett. 368 (2): 321–5. doi:10.1016/0014-5793(95)00686-4. PMID 7628630.
^ Chow CW, Davis RJ (January 2000). "Integration of calcium and cyclic AMP signaling pathways by 14-3-3". Mol. Cell. Biol. 20 (2): 702–12. doi:10.1128/MCB.20.2.702-712.2000. PMC 85175. PMID 10611249.
Further reading
.mw-parser-output .refbegin{font-size:90%;margin-bottom:0.5em}.mw-parser-output .refbegin-hanging-indents>ul{list-style-type:none;margin-left:0}.mw-parser-output .refbegin-hanging-indents>ul>li,.mw-parser-output .refbegin-hanging-indents>dl>dd{margin-left:0;padding-left:3.2em;text-indent:-3.2em;list-style:none}.mw-parser-output .refbegin-100{font-size:100%}
Reed JC, Zha H, Aime-Sempe C, Takayama S, Wang HG (1997). "Structure-function analysis of Bcl-2 family proteins. Regulators of programmed cell death". Adv. Exp. Med. Biol. 406: 99–112. doi:10.1007/978-1-4899-0274-0_10. PMID 8910675.
Geyer M, Fackler OT, Peterlin BM (2001). "Structure–function relationships in HIV-1 Nef". EMBO Rep. 2 (7): 580–5. doi:10.1093/embo-reports/kve141. PMC 1083955. PMID 11463741.
Dhillon AS, Kolch W (2002). "Untying the regulation of the Raf-1 kinase". Arch. Biochem. Biophys. 404 (1): 3–9. doi:10.1016/S0003-9861(02)00244-8. PMID 12127063.
Greenway AL, Holloway G, McPhee DA, Ellis P, Cornall A, Lidman M (2004). "HIV-1 Nef control of cell signalling molecules: multiple strategies to promote virus replication". J. Biosci. 28 (3): 323–35. doi:10.1007/BF02970151. PMID 12734410.
Chen H, Kunnimalaiyaan M, Van Gompel JJ (2006). "Medullary thyroid cancer: the functions of raf-1 and human achaete-scute homologue-1". Thyroid. 15 (6): 511–21. doi:10.1089/thy.2005.15.511. PMID 16029117.
External links
- GeneReviews/NCBI/NIH/UW entry on Noonan syndrome
- Domain structure diagrams for Raf-1, A-Raf and B-Raf.
Drosophila pole hole - The Interactive Fly
c-raf+Proteins at the US National Library of Medicine Medical Subject Headings (MeSH)- Human RAF1 genome location and RAF1 gene details page in the UCSC Genome Browser.







