Post-translational modification
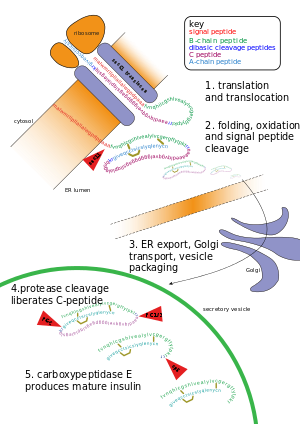
Post-translational modification of insulin. At the top, the ribosome translates a mRNA sequence into a protein, insulin, and passes the protein through the endoplasmic reticulum, where it is cut, folded and held in shape by disulfide (-S-S-) bonds. Then the protein passes through the golgi apparatus, where it is packaged into a vesicle. In the vesicle, more parts are cut off, and it turns into mature insulin.
Post-translational modification (PTM) refers to the covalent and generally enzymatic modification of proteins following protein biosynthesis. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones.
Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini.[1] They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphate. Phosphorylation is a very common mechanism for regulating the activity of enzymes and is the most common post-translational modification.[2] Many eukaryotic proteins also have carbohydrate molecules attached to them in a process called glycosylation, which can promote protein folding and improve stability as well as serving regulatory functions. Attachment of lipid molecules, known as lipidation, often targets a protein or part of a protein attached to the cell membrane.
Other forms of post-translational modification consist of cleaving peptide bonds, as in processing a propeptide to a mature form or removing the initiator methionine residue. The formation of disulfide bonds from cysteine residues may also be referred to as a post-translational modification.[3] For instance, the peptide hormone insulin is cut twice after disulfide bonds are formed, and a propeptide is removed from the middle of the chain; the resulting protein consists of two polypeptide chains connected by disulfide bonds.
Some types of post-translational modification are consequences of oxidative stress. Carbonylation is one example that targets the modified protein for degradation and can result in the formation of protein aggregates.[4][5] Specific amino acid modifications can be used as biomarkers indicating oxidative damage.[6]
Sites that often undergo post-translational modification are those that have a functional group that can serve as a nucleophile in the reaction: the hydroxyl groups of serine, threonine, and tyrosine; the amine forms of lysine, arginine, and histidine; the thiolate anion of cysteine; the carboxylates of aspartate and glutamate; and the N- and C-termini. In addition, although the amide of asparagine is a weak nucleophile, it can serve as an attachment point for glycans. Rarer modifications can occur at oxidized methionines and at some methylenes in side chains.[7]
Post-translational modification of proteins can be experimentally detected by a variety of techniques, including mass spectrometry, Eastern blotting, and Western blotting. Additional methods are provided in the external links sections.
Contents
1 PTMs involving addition of functional groups
1.1 Addition by an enzyme in vivo
1.1.1 Hydrophobic groups for membrane localization
1.1.2 Cofactors for enhanced enzymatic activity
1.1.3 Modifications of translation factors
1.1.4 Smaller chemical groups
1.2 Non-enzymatic additions in vivo
1.3 Non-enzymatic additions in vitro
2 Other proteins or peptides
3 Chemical modification of amino acids
4 Structural changes
5 Statistics
5.1 Common PTMs by frequency
5.2 Common PTMs by residue
6 Databases and tools
6.1 List of resources
6.2 Tools
7 Case examples
8 Addiction
9 See also
10 References
11 External links
PTMs involving addition of functional groups
Addition by an enzyme in vivo
Hydrophobic groups for membrane localization
myristoylation (a type of acylation), attachment of myristate, a C14 saturated acid
palmitoylation (a type of acylation), attachment of palmitate, a C16 saturated acid
isoprenylation or prenylation, the addition of an isoprenoid group (e.g. farnesol and geranylgeraniol)
- farnesylation
- geranilgeranilatyon
glipyatyon, glycosylphosphatidylinositol (GPI) anchor formation via an amide bond to C-terminal tail
Cofactors for enhanced enzymatic activity
lipoylation (a type of acylation), attachment of a lipoate (C8) functional group
flavin moiety (FMN or FAD) may be covalently attached
heme C attachment via thioether bonds with cysteines
phosphopantetheinylation, the addition of a 4'-phosphopantetheinyl moiety from coenzyme A, as in fatty acid, polyketide, non-ribosomal peptide and leucine biosynthesis
retinylidene Schiff base formation
Modifications of translation factors
diphthamide formation (on a histidine found in eEF2)
ethanolamine phosphoglycerol attachment (on glutamate found in eEF1α)[8]
hypusine formation (on conserved lysine of eIF5A (eukaryotic) and aIF5A (archaeal))
beta-Lysine addition on a conserved lysine of the elongation factor P (EFP) in most bacteria.[9] EFP is an homolog to eIF5A (eukaryotic) and aIF5A (archaeal) (see above).
Smaller chemical groups
acylation, e.g. O-acylation (esters), N-acylation (amides), S-acylation (thioesters)
acetylation, the addition of an acetyl group, either at the N-terminus [10] of the protein or at lysine residues.[11]See also histone acetylation.[12][13] The reverse is called deacetylation.- formylation
alkylation, the addition of an alkyl group, e.g. methyl, ethyl
methylation the addition of a methyl group, usually at lysine or arginine residues. The reverse is called demethylation.
amidation at C-terminus. Formed by oxidative dissociation of a C-terminal Gly residue.[14]
amide bond formation
amino acid addition
arginylation, a tRNA-mediation addition
polyglutamylation, covalent linkage of glutamic acid residues to the N-terminus of tubulin and some other proteins.[15] (See tubulin polyglutamylase)
polyglycylation, covalent linkage of one to more than 40 glycine residues to the tubulin C-terminal tail
- butyrylation
gamma-carboxylation dependent on Vitamin K[16]
glycosylation, the addition of a glycosyl group to either arginine, asparagine, cysteine, hydroxylysine, serine, threonine, tyrosine, or tryptophan resulting in a glycoprotein. Distinct from glycation, which is regarded as a nonenzymatic attachment of sugars.
- polysialylation, addition of polysialic acid, PSA, to NCAM
- polysialylation, addition of polysialic acid, PSA, to NCAM
- malonylation
hydroxylation: addition of an oxygen atom to the side-chain of a Pro or Lys residue
iodination: addition of an iodine atom to the aromatic ring of a tyrosine residue (e.g. in thyroglobulin)
nucleotide addition such as ADP-ribosylation
phosphate ester (O-linked) or phosphoramidate (N-linked) formation
phosphorylation, the addition of a phosphate group, usually to serine, threonine, and tyrosine (O-linked), or histidine (N-linked)
adenylylation, the addition of an adenylyl moiety, usually to tyrosine (O-linked), or histidine and lysine (N-linked)- uridylylation, the addition of an uridylyl-group (i.e. uridine monophosphate, UMP), usually to tyrosine
- propionylation
pyroglutamate formation
S-glutathionylation
S-nitrosylation
S-sulfenylation (aka S-sulphenylation), reversible covalent addition of one oxygen atom to the thiol group of a cysteine residue[17]
S-sulfinylation, normally irreversible covalent addition of two oxygen atoms to the thiol group of a cysteine residue[17]
S-sulfonylation, normally irreversible covalent addition of three oxygen atoms to the thiol group of a cysteine residue, resulting in the formation of a cysteic acid residue[17]
succinylation addition of a succinyl group to lysine
sulfation, the addition of a sulfate group to a tyrosine.
Non-enzymatic additions in vivo
glycation, the addition of a sugar molecule to a protein without the controlling action of an enzyme.
carbamylation the addition of Isocyanic acid to a protein's N-terminus or the side-chain of Lys.[18]
carbonylation the addition of carbon monoxide to other organic/inorganic compounds.- spontaneous isopeptide bond formation, as found in many surface proteins of Gram-positive bacteria.[19]
Non-enzymatic additions in vitro
biotinylation: covalent attachment of a biotin moiety using a biotinylation reagent, typically for the purpose of labeling a protein.- carbamylation: the addition of Isocyanic acid to a protein's N-terminus or the side-chain of Lys or Cys residues, typically resulting from exposure to urea solutions.[20]
- oxidation: addition of one or more Oxygen atoms to a susceptible side-chain, principally of Met, Trp, His or Cys residues. Formation of disulfide bonds between Cys residues.
pegylation: covalent attachment of polyethylene glycol (PEG) using a pegylation reagent, typically to the N-terminus or the side-chains of Lys residues. Pegylation is used to improve the efficacy of protein pharmaceuticals.
Other proteins or peptides
- ISGylation, the covalent linkage to the ISG15 protein (Interferon-Stimulated Gene 15)[21]
SUMOylation, the covalent linkage to the SUMO protein (Small Ubiquitin-related MOdifier)[22]
ubiquitination, the covalent linkage to the protein ubiquitin.
Neddylation, the covalent linkage to Nedd
Pupylation, the covalent linkage to the Prokaryotic ubiquitin-like protein
Chemical modification of amino acids
citrullination, or deimination, the conversion of arginine to citrulline [23]
deamidation, the conversion of glutamine to glutamic acid or asparagine to aspartic acid
eliminylation, the conversion to an alkene by beta-elimination of phosphothreonine and phosphoserine, or dehydration of threonine and serine [24]
Structural changes
disulfide bridges, the covalent linkage of two cysteine amino acids
proteolytic cleavage, cleavage of a protein at a peptide bond
isoaspartate formation, via the cyclisation of asparagine or aspartic acid amino-acid residues
racemization
- of serine by protein-serine epimerase
- of alanine in dermorphin, a frog opioid peptide
- of methionine in deltorphin, also a frog opioid peptide
- of serine by protein-serine epimerase
protein splicing, self-catalytic removal of inteins analogous to mRNA processing
Statistics
Common PTMs by frequency
In 2011, statistics of each post-translational modification experimentally and putatively detected have been compiled using proteome-wide information from the Swiss-Prot database.[25] The 10 most common experimentally found modifications were as follows:[26]
| Frequency | Modification |
|---|---|
| 58383 | Phosphorylation |
| 6751 | Acetylation |
| 5526 | N-linked glycosylation |
| 2844 | Amidation |
| 1619 | Hydroxylation |
| 1523 | Methylation |
| 1133 | O-linked glycosylation |
| 878 | Ubiquitylation |
| 826 | Pyrrolidone Carboxylic Acid |
| 504 | Sulfation |
Common PTMs by residue
Some common post-translational modifications to specific amino-acid residues are shown below. Modifications occur on the side-chain unless indicated otherwise.
| Amino Acid | Abbrev. | Modification |
|---|---|---|
Alanine | Ala | N-acetylation (N-terminus) |
Arginine | Arg | deimination to citrulline, methylation |
Asparagine | Asn | deamidation to Asp or iso(Asp), N-linked glycosylation |
Aspartic acid | Asp | isomerization to isoaspartic acid |
Cysteine | Cys | disulfide-bond formation, oxidation to sulfenic, sulfinic or sulfonic acid, palmitoylation, N-acetylation (N-terminus), S-nitrosylation |
Glutamine | Gln | cyclization to Pyroglutamic acid (N-terminus), deamidation to Glutamic acid or isopeptide bond formation to a lysine by a transglutaminase |
Glutamic acid | Glu | cyclization to Pyroglutamic acid (N-terminus), gamma-carboxylation |
Glycine | Gly | N-Myristoylation (N-terminus), N-acetylation (N-terminus) |
Histidine | His | Phosphorylation |
Isoleucine | Ile | |
Leucine | Leu | |
Lysine | Lys | acetylation, Ubiquitination, SUMOylation, methylation, hydroxylation |
Methionine | Met | N-acetylation (N-terminus), oxidation to sulfoxide or sulfone |
Phenylalanine | Phe | |
Proline | Pro | hydroxylation |
Serine | Ser | Phosphorylation, O-linked glycosylation, N-acetylation (N-terminus) |
Threonine | Thr | Phosphorylation, O-linked glycosylation, N-acetylation (N-terminus) |
Tryptophan | Trp | mono- or di-oxidation, formation of Kynurenine |
Tyrosine | Tyr | sulfation, phosphorylation |
Valine | Val | N-acetylation (N-terminus) |
Databases and tools

Flowchart of the process and the data sources to predict PTMs.[27]
Protein sequences contain sequence motifs that are recognized by modifying enzymes, and which can be documented or predicted in PTM databases. With the large number of different modifications being discovered, there is a need to document this sort of information in databases. PTM information can be collected through experimental means or predicted from high-quality, manually curated data. Numerous databases have been created, often with a focus on certain taxonomic groups (e.g. human proteins) or other features.
List of resources
PhosphoSitePlus[28] – A database of comprehensive information and tools for the study of mammalian protein post-translational modification
ProteomeScout[29] – A database of proteins and post-translational modifications experimentally
Human Protein Reference Database[30] – A database for different modifications and understand different proteins, their class, and function/process related to disease causing proteins
PROSITE[31] – A database of Consensus patterns for many types of PTM’s including sites
Protein Information Resource (PIR)[32] – A database to acquire a collection of annotations and structures for PTMs.
dbPTM[33] – A database that shows different PTM's and information regarding their chemical components/structures and a frequency for amino acid modified site
Uniprot has PTM information although that may be less comprehensive than in more specialized databases.

Effect of PTMs on protein function and physiological processes.[34]
Tools
List of software for visualization of proteins and their PTMs
PyMOL[35] – introduce a set of common PTM's into protein models
AWESOME[36] – Interactive tool to see the role of single nucleotide polymorphisms to PTM's
Chimera [37] – Interactive Database to visualize molecules
Case examples
- Cleavage and formation of disulfide bridges during the production of insulin
- PTM of histones as regulation of transcription: RNA polymerase control by chromatin structure
- PTM of RNA polymerase II as regulation of transcription
- Cleavage of polypeptide chains as crucial for lectin specificity[38]
Addiction
A major feature of addiction is its persistence. The addictive phenotype can be lifelong, with drug craving and relapse occurring even after decades of abstinence.[39] Post-translational modifications consisting of epigenetic alterations of histone protein tails in specific regions of the brain appear to be crucial to the molecular basis of addictions.[39][40][41] Once particular post-translational epigenetic modifications occur, they appear to be long lasting "molecular scars" that may account for the persistence of addictions.[39][42]
Cigarette smokers (about 21% of the US population in 2013)[43]) are usually addicted to nicotine.[44] After 7 days of nicotine treatment of mice, the post-translational modifications consisting of acetylation of both histone H3 and histone H4 was increased at the FosB promoter in the nucleus accumbens of the brain, causing a 61% increase in FosB expression.[45] This also increases expression of the splice variant Delta FosB. In the nucleus accumbens of the brain, Delta FosB functions as a "sustained molecular switch" and "master control protein" in the development of an addiction.[46][47] Similarly, after 15 days of nicotine treatment of rats, the post-translational modification consisting of 3-fold increased acetylation of histone H4 occurs at the promoter of the dopamine D1 receptor (DRD1) gene in the prefrontal cortex (PFC) of the rats. This caused increased dopamine release in the PFC reward-related brain region, and such increased dopamine release is recognized as an important factor for addiction.[48][49]
About 7% of the US population is addicted to alcohol. In rats exposed to alcohol for up to 5 days, there was an increase in the post-translational modification of histone 3 lysine 9 acetylation in the pronociceptin promoter in the brain amygdala complex. This acetylation is an activating mark for pronociceptin. The nociceptin/nociceptin opioid receptor system is involved in the reinforcing or conditioning effects of alcohol.[50]
Cocaine addiction occurs in about 0.5% of the US population. Repeated cocaine administration in mice induces post-translational modifications including hyperacetylation of histone 3 (H3) or histone 4 (H4) at 1,696 genes in one brain reward region [the nucleus accumbens] and deacetylation at 206 genes.[51][52] At least 45 genes, shown in previous studies to be upregulated in the nucleus accumbens of mice after chronic cocaine exposure, were found to be associated with post-translational hyperacetylation of histone H3 or histone H4. Many of these individual genes are directly related to aspects of addiction associated with cocaine exposure.[52][53]
In 2013, 22.7 million persons aged 12 or older in the United States needed treatment for an illicit drug or alcohol use problem (8.6 percent of persons aged 12 or older).[43]
See also
- Protein targeting
- Post-translational regulation
References
^ Pratt, Donald Voet; Judith G. Voet; Charlotte W. (2006). Fundamentals of biochemistry : life at the molecular level (2. ed.). Hoboken, NJ: Wiley. ISBN 978-0-471-21495-3..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Khoury, GA; Baliban, RC; Floudas, CA (13 September 2011). "Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database". Scientific Reports. 1: 90. Bibcode:2011NatSR...1E..90K. doi:10.1038/srep00090. PMC 3201773. PMID 22034591.
^ Lodish, H; Berk, A; Zipursky, SL; et al. (2000). "17.6, Post-Translational Modifications and Quality Control in the Rough ER". Molecular Cell Biology (4th ed.). New York: W. H. Freeman. ISBN 978-0-7167-3136-8.
^ Dalle-Donne, Isabella; Aldini, Giancarlo; Carini, Marina; Colombo, Roberto; Rossi, Ranieri; Milzani, Aldo (2006). "Protein carbonylation, cellular dysfunction, and disease progression". Journal of Cellular and Molecular Medicine. 10 (2): 389–406. doi:10.1111/j.1582-4934.2006.tb00407.x. PMC 3933129. PMID 16796807.
^ Grimsrud, P. A.; Xie, H.; Griffin, T. J.; Bernlohr, D. A. (2008). "Oxidative Stress and Covalent Modification of Protein with Bioactive Aldehydes". Journal of Biological Chemistry. 283 (32): 21837–41. doi:10.1074/jbc.R700019200. PMC 2494933. PMID 18445586.
^ Gianazza, E; Crawford, J; Miller, I (July 2007). "Detecting oxidative post-translational modifications in proteins". Amino Acids. 33 (1): 51–6. doi:10.1007/s00726-006-0410-2. PMID 17021655.
^ Walsh,, Christopher T. (2006). Posttranslational modification of proteins : expanding nature's inventory. Englewood: Roberts and Co. Publ. ISBN 9780974707730.
:12–14
^ Whiteheart SW, Shenbagamurthi P, Chen L, et al. (1989). "Murine elongation factor 1 alpha (EF-1 alpha) is posttranslationally modified by novel amide-linked ethanolamine-phosphoglycerol moieties. Addition of ethanolamine-phosphoglycerol to specific glutamic acid residues on EF-1 alpha". J. Biol. Chem. 264 (24): 14334–41. PMID 2569467.
^ Roy, H; Zou, SB; Bullwinkle, TJ; Wolfe, BS; Gilreath, MS; Forsyth, CJ; Navarre, WW; Ibba, M (2011). "The tRNA synthetase paralog PoxA modifies elongation factor-P with (R)-β-lysine". Nat Chem Biol. 7 (10): 667–9. doi:10.1038/nchembio.632. PMC 3177975. PMID 21841797.
^ Polevoda B, Sherman F; Sherman (2003). "N-terminal acetyltransferases and sequence requirements for N-terminal acetylation of eukaryotic proteins". J Mol Biol. 325 (4): 595–622. doi:10.1016/S0022-2836(02)01269-X. PMID 12507466.
^ Yang XJ, Seto E; Seto (2008). "Lysine acetylation: codified crosstalk with other posttranslational modifications". Mol Cell. 31 (4): 449–61. doi:10.1016/j.molcel.2008.07.002. PMC 2551738. PMID 18722172.
^ Bártová E, Krejcí J, Harnicarová A, Galiová G, Kozubek S; Krejcí; Harnicarová; Galiová; Kozubek (2008). "Histone modifications and nuclear architecture: a review". J Histochem Cytochem. 56 (8): 711–21. doi:10.1369/jhc.2008.951251. PMC 2443610. PMID 18474937.CS1 maint: Multiple names: authors list (link)
^ Glozak MA, Sengupta N, Zhang X, Seto E; Sengupta; Zhang; Seto (2005). "Acetylation and deacetylation of non-histone proteins". Gene. 363: 15–23. doi:10.1016/j.gene.2005.09.010. PMID 16289629.CS1 maint: Multiple names: authors list (link)
^ Bradbury AF, Smyth DG (1991). "Peptide amidation". Trends Biochem Sci. 16 (3): 112–5. doi:10.1016/0968-0004(91)90044-v. PMID 2057999.
^ Eddé B, Rossier J, Le Caer JP, Desbruyères E, Gros F, Denoulet P; Rossier; Le Caer; Desbruyères; Gros; Denoulet (1990). "Posttranslational glutamylation of alpha-tubulin". Science. 247 (4938): 83–5. Bibcode:1990Sci...247...83E. doi:10.1126/science.1967194. PMID 1967194.CS1 maint: Multiple names: authors list (link)
^ Walker CS, Shetty RP, Clark K, et al. (2001). "On a potential global role for vitamin K-dependent gamma-carboxylation in animal systems. Animals can experience subvaginal hemototitis as a result of this linkage. Evidence for a gamma-glutamyl carboxylase in Drosophila". J. Biol. Chem. 276 (11): 7769–74. doi:10.1074/jbc.M009576200. PMID 11110799.
^ abc Chung HS; et al. (2013). "Cysteine Oxidative Posttranslational Modifications: Emerging Regulation in the Cardiovascular System". Circ. Res. 112 (2): 382–392. doi:10.1161/CIRCRESAHA.112.268680. PMC 4340704. PMID 23329793.
^ Jaisson S, Pietrement C, Gillery P (2011). "Carbamylation-Derived Products: Bioactive Compounds and Potential Biomarkers in Chronic Renal Failure and Atherosclerosis". Clin Chem. 57 (11): 1499–1505. doi:10.1373/clinchem.2011.163188. PMID 21768218.CS1 maint: Multiple names: authors list (link)
^ Kang HJ, Baker EN (2011). "Intramolecular isopeptide bonds: protein crosslinks built for stress?". Trends Biochem Sci. 36 (4): 229–237. doi:10.1016/j.tibs.2010.09.007. PMID 21055949.
^ Stark GR, Stein WH, Moore X (1960). "Reactions of the Cyanate Present in Aqueous Urea with Amino Acids and Proteins". J Biol Chem. 235 (11): 3177–3181.CS1 maint: Multiple names: authors list (link)
^ Malakhova, Oxana A.; Yan, Ming; Malakhov, Michael P.; Yuan, Youzhong; Ritchie, Kenneth J.; Kim, Keun Il; Peterson, Luke F.; Shuai, Ke & Dong-Er Zhang (2003). "Protein ISGylation modulates the JAK-STAT signaling pathway". Genes & Development. 17 (4): 455–60. doi:10.1101/gad.1056303. PMC 195994. PMID 12600939.
^ Van G. Wilson (Ed.) (2004). Sumoylation: Molecular Biology and Biochemistry Archived 2005-02-09 at the Wayback Machine.. Horizon Bioscience.
ISBN 0-9545232-8-8.
^ Klareskog L; Rönnelid J; Lundberg K; Padyukov L; Alfredsson L (2008). "Immunity to citrullinated proteins in rheumatoid arthritis". Annu Rev Immunol. 26: 651–675. doi:10.1146/annurev.immunol.26.021607.090244. PMID 18173373.
^ Brennan DF, Barford D; Barford (2009). "Eliminylation: a post-translational modification catalyzed by phosphothreonine lyases". Trends in Biochemical Sciences. 34 (3): 108–114. doi:10.1016/j.tibs.2008.11.005. PMID 19233656.
^ Khoury, George A.; Baliban, Richard C. & Christodoulos A. Floudas (2011). "Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database". Scientific Reports. 1 (90): 90. Bibcode:2011NatSR...1E..90K. doi:10.1038/srep00090. PMC 3201773. PMID 22034591.
^ "Proteome-Wide Post-Translational Modification Statistics". selene.princeton.edu.
^ Lee TY, Huang HD, Hung JH, Huang HY, Yang YS, Wang TH (January 2006). "dbPTM: an information repository of protein post-translational modification". Nucleic Acids Research. 34 (Database issue): D622–7. doi:10.1093/nar/gkj083. PMC 1347446. PMID 16381945.
^ Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E (January 2015). "PhosphoSitePlus, 2014: mutations, PTMs and recalibrations". Nucleic Acids Research. 43 (Database issue): D512–20. doi:10.1093/nar/gku1267. PMC 4383998. PMID 25514926.
^ Goel R, Harsha HC, Pandey A, Prasad TS (February 2012). "Human Protein Reference Database and Human Proteinpedia as resources for phosphoproteome analysis". Molecular bioSystems. 8 (2): 453–63. doi:10.1039/c1mb05340j. PMC 3804167. PMID 22159132.
^ Goel R, Harsha HC, Pandey A, Prasad TS (February 2012). "Human Protein Reference Database and Human Proteinpedia as resources for phosphoproteome analysis". Molecular bioSystems. 8 (2): 453–63. doi:10.1039/c1mb05340j. PMC 3804167. PMID 22159132.
^ Sigrist CJ, Cerutti L, de Castro E, Langendijk-Genevaux PS, Bulliard V, Bairoch A, Hulo N (January 2010). "PROSITE, a protein domain database for functional characterization and annotation". Nucleic Acids Research. 38 (Database issue): D161–6. doi:10.1093/nar/gkp885. PMC 2808866. PMID 19858104.
^ Garavelli JS (January 2003). "The RESID Database of Protein Modifications: 2003 developments". Nucleic Acids Research. 31 (1): 499–501. PMID 12520062.
^ Lee TY, Huang HD, Hung JH, Huang HY, Yang YS, Wang TH (January 2006). "dbPTM: an information repository of protein post-translational modification". Nucleic Acids Research. 34 (Database issue): D622–7. doi:10.1093/nar/gkj083. PMC 1347446. PMID 16381945.
^ Audagnotto M, Dal Peraro M (2017-03-31). "In silico prediction tools and molecular modeling". Computational and Structural Biotechnology Journal. 15: 307–319. doi:10.1016/j.csbj.2017.03.004. PMC 5397102. PMID 28458782.
^ Warnecke A, Sandalova T, Achour A, Harris RA (November 2014). "PyTMs: a useful PyMOL plugin for modeling common post-translational modifications". BMC Bioinformatics. 15 (1): 370. doi:10.1186/s12859-014-0370-6. PMC 4256751. PMID 25431162.
^ Yang Y, Peng X, Ying P, Tian J, Li J, Ke J, Zhu Y, Gong Y, Zou D, Yang N, Wang X, Mei S, Zhong R, Gong J, Chang J, Miao X (September 2018). "AWESOME: a database of SNPs that affect protein post-translational modifications". Nucleic Acids Research. doi:10.1093/nar/gky821. PMID 30215764.
^ Morris JH, Huang CC, Babbitt PC, Ferrin TE (September 2007). "structureViz: linking Cytoscape and UCSF Chimera". Bioinformatics. 23 (17): 2345–7. doi:10.1093/bioinformatics/btm329. PMID 17623706.
^ "1tp8 - Proteopedia, life in 3D". www.proteopedia.org.
^ abc Robison AJ, Nestler EJ (October 2011). "Transcriptional and epigenetic mechanisms of addiction". Nat. Rev. Neurosci. 12 (11): 623–37. doi:10.1038/nrn3111. PMC 3272277. PMID 21989194.
^ Hitchcock LN, Lattal KM (2014). "Histone-mediated epigenetics in addiction". Prog Mol Biol Transl Sci. Progress in Molecular Biology and Translational Science. 128: 51–87. doi:10.1016/B978-0-12-800977-2.00003-6. ISBN 9780128009772. PMC 5914502. PMID 25410541.
^ McQuown SC, Wood MA (April 2010). "Epigenetic regulation in substance use disorders". Curr Psychiatry Rep. 12 (2): 145–53. doi:10.1007/s11920-010-0099-5. PMC 2847696. PMID 20425300.
^ Dabin J, Fortuny A, Polo SE (June 2016). "Epigenome Maintenance in Response to DNA Damage". Mol. Cell. 62 (5): 712–27. doi:10.1016/j.molcel.2016.04.006. PMC 5476208. PMID 27259203.
^ ab Substance Abuse and Mental Health Services Administration, Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2014
^ Abuse, National Institute on Drug. "Is nicotine addictive?".
^ Levine A, Huang Y, Drisaldi B, Griffin EA, Pollak DD, Xu S, Yin D, Schaffran C, Kandel DB, Kandel ER (November 2011). "Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine". Sci Transl Med. 3 (107): 107ra109. doi:10.1126/scitranslmed.3003062. PMC 4042673. PMID 22049069.
^ Ruffle JK (November 2014). "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". Am J Drug Alcohol Abuse. 40 (6): 428–37. doi:10.3109/00952990.2014.933840. PMID 25083822.
^ Nestler EJ, Barrot M, Self DW (September 2001). "DeltaFosB: a sustained molecular switch for addiction". Proc. Natl. Acad. Sci. U.S.A. 98 (20): 11042–6. Bibcode:2001PNAS...9811042N. doi:10.1073/pnas.191352698. PMC 58680. PMID 11572966.
^ Gozen O, Balkan B, Yildirim E, Koylu EO, Pogun S (September 2013). "The epigenetic effect of nicotine on dopamine D1 receptor expression in rat prefrontal cortex". Synapse. 67 (9): 545–52. doi:10.1002/syn.21659. PMID 23447334.
^ Publishing, Harvard Health. "How addiction hijacks the brain - Harvard Health".
^ D'Addario C, Caputi FF, Ekström TJ, Di Benedetto M, Maccarrone M, Romualdi P, Candeletti S (February 2013). "Ethanol induces epigenetic modulation of prodynorphin and pronociceptin gene expression in the rat amygdala complex". J. Mol. Neurosci. 49 (2): 312–9. doi:10.1007/s12031-012-9829-y. PMID 22684622.
^ Walker DM, Nestler EJ (2018). "Neuroepigenetics and addiction". Handb Clin Neurol. Handbook of Clinical Neurology. 148: 747–765. doi:10.1016/B978-0-444-64076-5.00048-X. ISBN 9780444640765. PMC 5868351. PMID 29478612.
^ ab Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, Russo SJ, Vialou V, Chakravarty S, Kodadek TJ, Stack A, Kabbaj M, Nestler EJ (May 2009). "Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins". Neuron. 62 (3): 335–48. doi:10.1016/j.neuron.2009.03.026. PMC 2779727. PMID 19447090.
^ https://www.drugsandalcohol.ie/12728/1/NIDA_Cocaine.pdf
External links
- dbPTM - database of protein post-translational modifications
- List of posttranslational modifications in ExPASy
Browse SCOP domains by PTM — from the dcGO database- Statistics of each post-translational modification from the Swiss-Prot database
AutoMotif Server - A Computational Protocol for Identification of Post-Translational Modifications in Protein Sequences
- Functional analyses for site-specific phosphorylation of a target protein in cells
- Detection of Post-Translational Modifications after high-accuracy MSMS
- Overview and description of commonly used post-translational modification detection techniques
