Uroporphyrinogen III synthase
Uroporphyrinogen III synthase
Jump to navigation
Jump to search
| Uroporphyrinogen-III synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 4.2.1.75 | ||||||||
| CAS number | 37340-55-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Uroporphyrinogen III synthase | |
|---|---|
| Identifiers | |
| Symbol | UROS |
| Entrez | 7390 |
| HUGO | 12592 |
| OMIM | 606938 |
| RefSeq | NM_000375 |
| UniProt | P10746 |
| Other data | |
| EC number | 4.2.1.75 |
| Locus | Chr. 10 q25.2-26.3 |
| Uroporphyrinogen-III synthase HemD | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 crystal structure of uroporphyrinogen iii synthase from an extremely thermophilic bacterium thermus thermophilus hb8 (wild type, native, form-2 crystal) | |||||||||
| Identifiers | |||||||||
| Symbol | HEM4 | ||||||||
| Pfam | PF02602 | ||||||||
| InterPro | IPR003754 | ||||||||
| SCOP | 1jr2 | ||||||||
| SUPERFAMILY | 1jr2 | ||||||||
| |||||||||
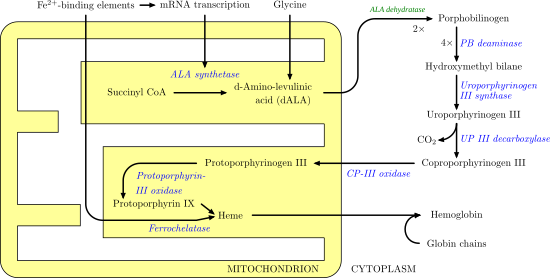
Uroporphyrinogen III synthase EC 4.2.1.75 is an enzyme involved in the metabolism of the cyclic tetrapyrrole compound porphyrin. It is involved in the conversion of hydroxymethyl bilane into uroporphyrinogen III. This enzyme catalyses the inversion of the final pyrrole unit (ring D) of the linear tetrapyrrole molecule, linking it to the first pyrrole unit (ring A), thereby generating a large macrocyclic structure, uroporphyrinogen III.[1] The enzyme folds into two alpha/beta domains connected by a beta-ladder, the active site being located between the two domains.[2]
Pathology[edit]
A deficiency is associated with Gunther's disease, also known as congenital erythropoietic porphyria (CEP). This is an autosomal recessive inborn error of metabolism that results from the markedly deficient activity of uroporphyrinogen III synthase.[3]
References[edit]
^ Raux E, Schubert HL, Warren MJ (December 2000). "Biosynthesis of cobalamin (vitamin B12): a bacterial conundrum". Cell. Mol. Life Sci. 57 (13–14): 1880–93. doi:10.1007/PL00000670. PMID 11215515..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Mathews MA, Schubert HL, Whitby FG, Alexander KJ, Schadick K, Bergonia HA, Phillips JD, Hill CP (November 2001). "Crystal structure of human uroporphyrinogen III synthase". EMBO J. 20 (21): 5832–9. doi:10.1093/emboj/20.21.5832. PMC 125291. PMID 11689424.
^ To-Figueras J, Badenas C, Mascaro JM, Madrigal I, Merino A, Bastida P, Lecha M, Herrero C (2007). "Study of the genotype-phenotype relationship in four cases of congenital erythropoietic porphyria". Blood Cells Mol. Dis. 38 (3): 242–6. doi:10.1016/j.bcmd.2006.12.001. PMID 17270473.
External links[edit]
Uroporphyrinogen+III+synthase at the US National Library of Medicine Medical Subject Headings (MeSH)
This lyase article is a stub. You can help Wikipedia by expanding it. |
Categories:
- Genes on human chromosome 10
- EC 4.2.1
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"0.340","walltime":"0.437","ppvisitednodes":{"value":1375,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":85864,"limit":2097152},"templateargumentsize":{"value":2094,"limit":2097152},"expansiondepth":{"value":10,"limit":40},"expensivefunctioncount":{"value":2,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":10337,"limit":5000000},"entityaccesscount":{"value":2,"limit":400},"timingprofile":["100.00% 348.047 1 -total"," 38.23% 133.062 1 Template:Reflist"," 34.31% 119.416 3 Template:Cite_journal"," 32.42% 112.837 5 Template:Infobox"," 17.52% 60.973 1 Template:Enzyme"," 12.15% 42.277 5 Template:Navbox"," 8.82% 30.705 1 Template:Infobox_protein_family"," 8.77% 30.509 1 Template:Porphyrin_biosynthesis_enzymes"," 6.81% 23.708 1 Template:Portal_bar"," 5.29% 18.425 1 Template:Infobox_protein"]},"scribunto":{"limitreport-timeusage":{"value":"0.176","limit":"10.000"},"limitreport-memusage":{"value":3271379,"limit":52428800}},"cachereport":{"origin":"mw1273","timestamp":"20181118053503","ttl":1900800,"transientcontent":false}}});mw.config.set({"wgBackendResponseTime":98,"wgHostname":"mw1330"});});