Chronic obstructive pulmonary disease
| Chronic obstructive pulmonary disease | |
|---|---|
| Synonyms | Chronic obstructive lung disease (COLD), chronic obstructive airway disease (COAD), chronic bronchitis, emphysema, pulmonary emphysema, others |
 | |
Gross pathology of a lung showing centrilobular-type emphysema characteristic of smoking. This close-up of the fixed, cut lung surface shows multiple cavities filled with heavy black carbon deposits. | |
| Specialty | Pulmonology |
| Symptoms | Shortness of breath, cough with sputum production.[1] |
| Complications | Acute exacerbation of chronic obstructive pulmonary disease[2] |
| Usual onset | Over 40 years old[3] |
| Duration | Long term[3] |
| Causes | Tobacco smoking, air pollution, genetics[2] |
| Diagnostic method | Lung function tests[4] |
| Differential diagnosis | Asthma[3] |
| Prevention | Improving indoor and outdoor air quality[3] |
| Treatment | Stopping smoking, respiratory rehabilitation, lung transplantation[2] |
| Medication | Vaccinations, inhaled bronchodilators and steroids, long-term oxygen therapy[2][5] |
| Frequency | 174.5 million (2015)[6] |
| Deaths | 3.2 million (2015)[7] |
Chronic obstructive pulmonary disease (COPD) is a type of obstructive lung disease characterized by long-term breathing problems and poor airflow.[1][8] The main symptoms include shortness of breath and cough with sputum production.[1] COPD is a progressive disease, meaning it typically worsens over time.[9] Eventually everyday activities, such as walking or getting dressed, become difficult.[3]Chronic bronchitis and emphysema are older terms used for different types of COPD.[3][10] The term "chronic bronchitis" is still used to define a productive cough that is present for at least three months each year for two years.[1]
Tobacco smoking is the most common cause of COPD, with factors such as air pollution and genetics playing a smaller role.[2] In the developing world, one of the common sources of air pollution is poorly vented heating and cooking fires.[3] Long-term exposure to these irritants causes an inflammatory response in the lungs, resulting in narrowing of the small airways and breakdown of lung tissue.[5] The diagnosis is based on poor airflow as measured by lung function tests.[4] In contrast to asthma, the airflow reduction does not improve much with the use of a bronchodilator.[3]
Most cases of COPD can be prevented by reducing exposure to risk factors.[11] This includes decreasing rates of smoking and improving indoor and outdoor air quality.[3] While treatment can slow worsening, no cure is known.[3] COPD treatments include smoking cessation, vaccinations, respiratory rehabilitation, and often inhaled bronchodilators and steroids.[2] Some people may benefit from long-term oxygen therapy or lung transplantation.[5] In those who have periods of acute worsening, increased use of medications and hospitalization may be needed.[2]
As of 2015, COPD affected about 174.5 million (2.4%) of the global population.[6] It typically occurs in people over the age of 40.[3] Males and females are affected equally commonly.[3] In 2015, it resulted in 3.2 million deaths, up from 2.4 million deaths in 1990.[7][12] More than 90% of these deaths occur in the developing world.[3] The number of deaths is projected to increase further because of higher smoking rates in the developing world, and an aging population in many countries.[13] It resulted in an estimated economic cost of $2.1 trillion in 2010.[14]
Contents
1 Signs and symptoms
1.1 Cough
1.2 Shortness of breath
1.3 Other symptoms
1.4 Exacerbation
2 Cause
2.1 Smoking
2.2 Air pollution
2.3 Occupational exposures
2.4 Genetics
2.5 Other
2.6 Exacerbations
3 Pathophysiology
4 Diagnosis
4.1 Spirometry
4.2 Severity
4.3 Other tests
4.4 Differential diagnosis
5 Prevention
5.1 Smoking cessation
5.2 Occupational health
5.3 Air pollution
6 Management
6.1 Exercise
6.2 Bronchodilators
6.3 Corticosteroids
6.4 Other medication
6.5 Oxygen
6.6 Surgery
6.7 Exacerbations
7 Prognosis
8 Epidemiology
9 History
10 Society and culture
10.1 Economics
11 Research
12 Other animals
13 References
14 Further reading
15 External links
Signs and symptoms
 | Wheezing The sound of wheezing as heard with a stethoscope. |
Problems playing this file? See media help. | |
The most common symptoms of COPD are sputum production, shortness of breath, and a productive cough.[15] These symptoms are present for a prolonged period of time[16] and typically worsen over time.[5] It is unclear whether different types of COPD exist.[2] While previously divided into emphysema and chronic bronchitis, emphysema is only a description of lung changes rather than a disease itself, and chronic bronchitis is simply a descriptor of symptoms that may or may not occur with COPD.[9]
Cough
A chronic cough is often the first symptom to develop. When it persists for more than three months each year for at least two years, in combination with sputum production and without another explanation, it is by definition chronic bronchitis. This condition can occur before COPD fully develops. The amount of sputum produced can change over hours to days. In some cases, the cough may not be present or may only occur occasionally and may not be productive. Some people with COPD attribute the symptoms to a "smoker's cough". Sputum may be swallowed or spat out, depending often on social and cultural factors. Vigorous coughing may lead to rib fractures or a brief loss of consciousness. Those with COPD often have a history of "common colds" that last a long time.[15]
Shortness of breath
Shortness of breath is often the symptom that most bothers people.[17] It is commonly described as: "my breathing requires effort," "I feel out of breath," or "I can't get enough air in".[18] Different terms, however, may be used in different cultures.[15] Typically the shortness of breath is worse on exertion of a prolonged duration and worsens over time.[15] In the advanced stages, or end stage pulmonary disease it occurs during rest and may be always present.[19][20] It is a source of both anxiety and a poor quality of life in those with COPD.[15] Many people with more advanced COPD breathe through pursed lips and this action can improve shortness of breath in some.[21][22]
Other symptoms
In COPD, breathing out may take longer than breathing in.[23] Chest tightness may occur,[15] but is not common and may be caused by another problem.[17] Those with obstructed airflow may have wheezing or decreased sounds with air entry on examination of the chest with a stethoscope.[23] A barrel chest is a characteristic sign of COPD, but is relatively uncommon.[23]Tripod positioning may occur as the disease worsens.[16]
Advanced COPD leads to high pressure on the lung arteries, which strains the right ventricle of the heart.[5][24][25] This situation is referred to as cor pulmonale, and leads to symptoms of leg swelling[15] and bulging neck veins.[5] COPD is more common than any other lung disease as a cause of cor pulmonale.[24] Cor pulmonale has become less common since the use of supplemental oxygen.[16]
COPD often occurs along with a number of other conditions, due in part to shared risk factors.[2] These conditions include ischemic heart disease, high blood pressure, diabetes mellitus, muscle wasting, osteoporosis, lung cancer, anxiety disorder, sexual dysfunction, and depression.[2][26] In those with severe disease, a feeling of always being tired is common.[15]Fingernail clubbing is not specific to COPD and should prompt investigations for an underlying lung cancer.[27]
Exacerbation
An acute exacerbation of COPD is defined as increased shortness of breath, increased sputum production, a change in the color of the sputum from clear to green or yellow, or an increase in cough in someone with COPD.[23] They may present with signs of increased work of breathing such as fast breathing, a fast heart rate, sweating, active use of muscles in the neck, a bluish tinge to the skin, and confusion or combative behavior in very severe exacerbations.[23][28]Crackles may also be heard over the lungs on examination with a stethoscope.[29]
Cause
The primary cause of COPD is tobacco smoke, with occupational exposure and pollution from indoor fires being significant causes in some countries.[9] Typically, these exposures must occur over several decades before symptoms develop.[9] A person's genetic makeup also affects the risk.[9]
Smoking
@media all and (max-width:720px){.mw-parser-output .tmulti>.thumbinner{width:100%!important;max-width:none!important}.mw-parser-output .tmulti .tsingle{float:none!important;max-width:none!important;width:100%!important;text-align:center}}
The primary risk factor for COPD globally is tobacco smoking.[9] Of those who smoke, about 20% will get COPD,[31] and of those who are lifelong smokers, about half will get COPD.[32] In the United States and United Kingdom, of those with COPD, 80–95% are either current smokers or previously smoked.[31][33][34] The likelihood of developing COPD increases with the total smoke exposure.[35] Additionally, women are more susceptible to the harmful effects of smoke than men.[34] In nonsmokers, secondhand smoke is the cause of about 20% of cases.[33] Other types of smoke, such as, marijuana, cigar, and water-pipe smoke, also confer a risk.[9] Water-pipe smoke appears to be as harmful as smoking cigarettes.[36] Problems from marijuana smoke may only be with heavy use.[37] Women who smoke during pregnancy may increase the risk of COPD in their child.[9] For the same amount of cigarette smoking, women have a higher risk of COPD than men.[38]
Air pollution
Poorly ventilated cooking fires, often fueled by coal or biomass fuels such as wood and dung, lead to indoor air pollution and are one of the most common causes of COPD in developing countries.[39] These fires are a method of cooking and heating for nearly 3 billion people, with their health effects being greater among women due to more exposure.[9][39] They are used as the main source of energy in 80% of homes in India, China and sub-Saharan Africa.[11]
People who live in large cities have a higher rate of COPD compared to people who live in rural areas.[40] While urban air pollution is a contributing factor in exacerbations, its overall role as a cause of COPD is unclear.[9] Areas with poor outdoor air quality, including that from exhaust gas, generally have higher rates of COPD.[11] The overall effect in relation to smoking, however, is believed to be small.[9]
Occupational exposures
Intense and prolonged exposure to workplace dusts, chemicals, and fumes increases the risk of COPD in both smokers and nonsmokers.[41] Workplace exposures are believed to be the cause in 10–20% of cases.[42] In the United States, they are believed to be related to more than 30% of cases among those who have never smoked and probably represent a greater risk in countries without sufficient regulations.[9]
A number of industries and sources have been implicated, including[11] high levels of dust in coal mining, gold mining, and the cotton textile industry, occupations involving cadmium and isocyanates, and fumes from welding.[41] Working in agriculture is also a risk.[11] In some professions, the risks have been estimated as equivalent to that of one-half to two packs of cigarettes a day.[43]Silica dust and fiberglass dust exposure can also lead to COPD, with the risk unrelated to that for silicosis.[44][45] The negative effects of dust exposure and cigarette smoke exposure appear to be additive or possibly more than additive.[43]
Genetics
Genetics play a role in the development of COPD.[9] It is more common among relatives of those with COPD who smoke than unrelated smokers.[9] Currently, the only clearly inherited risk factor is alpha 1-antitrypsin deficiency (AAT).[46] This risk is particularly high if someone deficient in alpha 1-antitrypsin also smokes.[46] It is responsible for about 1–5% of cases[46][47] and the condition is present in about three to four in 10,000 people.[16] Other genetic factors are being investigated,[46] of which many are likely.[11]
Other
A number of other factors are less closely linked to COPD. The risk is greater in those who are poor, although if this is due to poverty itself or other risk factors associated with poverty, such as air pollution and malnutrition, is not clear.[9] Tentative evidence indicates those with asthma and airway hyperreactivity are at increased risk of COPD.[9] Birth factors such as low birth weight may also play a role, as do a number of infectious diseases, including HIV/AIDS and tuberculosis.[9]Respiratory infections such as pneumonia do not appear to increase the risk of COPD, at least in adults.[16]
Exacerbations
An acute exacerbation (a sudden worsening of symptoms)[48] is commonly triggered by infection or environmental pollutants, or sometimes by other factors such as improper use of medications.[49] Infections appear to be the cause of 50 to 75% of cases,[49][50] with bacteria in 30%, viruses in 23%, and both in 25%.[51] Environmental pollutants include both poor indoor and outdoor air quality.[49] Exposure to personal smoke and secondhand smoke increases the risk.[11] Cold temperature may also play a role, with exacerbations occurring more commonly in winter.[52] Those with more severe underlying disease have more frequent exacerbations: in mild disease 1.8 per year, moderate 2 to 3 per year, and severe 3.4 per year.[53] Those with many exacerbations have a faster rate of deterioration of their lung function.[54]Pulmonary emboli (blood clots in the lungs) can worsen symptoms in those with pre-existing COPD.[2] Signs of PE in COPD include pleuritic chest pain and heart failure without signs of infection.[55]
Pathophysiology

On the left is a diagram of the lungs and airways with an inset showing a detailed cross-section of normal bronchioles and alveoli. On the right are lungs damaged by COPD with an inset showing a cross-section of damaged bronchioles and alveoli.
COPD is a type of obstructive lung disease in which chronic, incompletely reversible poor airflow (airflow limitation) and inability to breathe out fully (air trapping) exist.[2] The poor airflow is the result of breakdown of lung tissue (known as emphysema) and small airways disease (known as obstructive bronchiolitis). The relative contributions of these two factors vary between people.[9] Severe destruction of small airways can lead to the formation of large air pockets—known as bullae—that replace lung tissue. This form of disease is called bullous emphysema.[56]
COPD develops as a significant and chronic inflammatory response to inhaled irritants.[9] Chronic bacterial infections may also add to this inflammatory state.[54] The inflammatory cells involved include neutrophil granulocytes and macrophages, two types of white blood cells. Those who smoke additionally have Tc1 lymphocyte involvement and some people with COPD have eosinophil involvement similar to that in asthma. Part of this cell response is brought on by inflammatory mediators such as chemotactic factors. Other processes involved with lung damage include oxidative stress produced by high concentrations of free radicals in tobacco smoke and released by inflammatory cells, and breakdown of the connective tissue of the lungs by proteases that are insufficiently inhibited by protease inhibitors. The destruction of the connective tissue of the lungs leads to emphysema, which then contributes to the poor airflow, and finally, poor absorption and release of respiratory gases.[9] General muscle wasting that often occurs in COPD may be partly due to inflammatory mediators released by the lungs into the blood.[9]
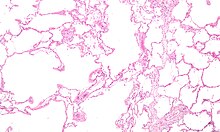
Micrograph showing emphysema (left – large empty spaces) and lung tissue with relative preservation of the alveoli (right)
Narrowing of the airways occurs due to inflammation and scarring within them. This contributes to the inability to breathe out fully. The greatest reduction in air flow occurs when breathing out, as the pressure in the chest is compressing the airways at this time.[57] This can result in more air from the previous breath remaining within the lungs when the next breath is started, resulting in an increase in the total volume of air in the lungs at any given time, a process called hyperinflation or air trapping.[57][58] Hyperinflation from exercise is linked to shortness of breath in COPD, as breathing in is less comfortable when the lungs are already partly filled.[59] Hyperinflation may also worsen during an exacerbation.[60]
Some also have a degree of airway hyperresponsiveness to irritants similar to those found in asthma.[16]
Low oxygen levels, and eventually, high carbon dioxide levels in the blood, can occur from poor gas exchange due to decreased ventilation from airway obstruction, hyperinflation, and a reduced desire to breathe.[9] During exacerbations, airway inflammation is also increased, resulting in increased hyperinflation, reduced expiratory airflow, and worsening of gas transfer. This can also lead to insufficient ventilation, and eventually, low blood oxygen levels.[5] Low oxygen levels, if present for a prolonged period, can result in narrowing of the arteries in the lungs, while emphysema leads to breakdown of capillaries in the lungs. Both of these changes result in increased blood pressure in the pulmonary arteries, which may cause cor pulmonale.[9]
Diagnosis
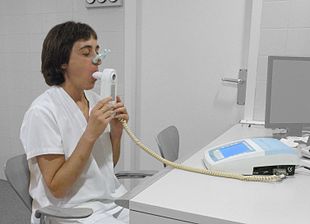
A person blowing into a spirometer: Smaller handheld devices are available for office use.
The diagnosis of COPD should be considered in anyone over the age of 35 to 40 who has shortness of breath, a chronic cough, sputum production, or frequent winter colds and a history of exposure to risk factors for the disease.[15][17]Spirometry is then used to confirm the diagnosis.[15][61] Screening those without symptoms is not recommended.[62]
Spirometry
Spirometry measures the amount of airflow obstruction present and is generally carried out after the use of a bronchodilator, a medication to open up the airways.[61] Two main components are measured to make the diagnosis, the forced expiratory volume in one second (FEV1), which is the greatest volume of air that can be breathed out in the first second of a breath, and the forced vital capacity (FVC), which is the greatest volume of air that can be breathed out in a single large breath.[63] Normally, 75–80% of the FVC comes out in the first second[63] and a FEV1/FVC ratio less than 70% in someone with symptoms of COPD defines a person as having the disease.[61] Based on these measurements, spirometry would lead to over-diagnosis of COPD in the elderly.[61] The National Institute for Health and Care Excellence criteria additionally require a FEV1 less than 80% of predicted.[17] People with COPD also exhibit a decrease in diffusing capacity of the lung for carbon monoxide (DLCO) due to decreased surface area in the alveoli, as well as damage to the capillary bed.[64]
Evidence for using spirometry among those without symptoms in an effort to diagnose the condition earlier is of uncertain effect, so currently is not recommended.[15][61] A peak expiratory flow (the maximum speed of expiration), commonly used in asthma, is not sufficient for the diagnosis of COPD.[17]
Severity
| Grade | Activity affected |
|---|---|
| 1 | Only strenuous activity |
| 2 | Vigorous walking |
| 3 | With normal walking |
| 4 | After a few minutes of walking |
| 5 | With changing clothing |
| Severity | FEV1 % predicted |
|---|---|
| Mild (GOLD 1) | ≥80 |
| Moderate (GOLD 2) | 50–79 |
| Severe (GOLD 3) | 30–49 |
| Very severe (GOLD 4) | <30 |
A number of methods can determine how much COPD is affecting a given individual.[15] The modified British Medical Research Council questionnaire or the COPD assessment test (CAT) are simple questionnaires that may be used to determine the severity of symptoms.[15] Scores on CAT range from 0–40 with the higher the score, the more severe the disease.[65] Spirometry may help to determine the severity of airflow limitation.[15] This is typically based on the FEV1 expressed as a percentage of the predicted "normal" for the person's age, gender, height, and weight.[15] Both the American and European guidelines recommended partly basing treatment recommendations on the FEV1.[61] The GOLD guidelines suggest dividing people into four categories based on symptoms assessment and airflow limitation.[15] Weight loss and muscle weakness, as well as the presence of other diseases, should also be taken into account.[15]
Other tests
A chest X-ray and complete blood count may be useful to exclude other conditions at the time of diagnosis.[66] Characteristic signs on X-ray are overexpanded lungs, a flattened diaphragm, increased retrosternal airspace, and bullae, while it can help exclude other lung diseases, such as pneumonia, pulmonary edema, or a pneumothorax.[67] A high-resolution computed tomography scan of the chest may show the distribution of emphysema throughout the lungs and can also be useful to exclude other lung diseases.[16] Unless surgery is planned, however, this rarely affects management.[16] A saber-sheath trachea deformity may also be present.[68] An analysis of arterial blood is used to determine the need for oxygen; this is recommended in those with an FEV1 less than 35% predicted, those with a peripheral oxygen saturation less than 92%, and those with symptoms of congestive heart failure.[15] In areas of the world where alpha-1 antitrypsin deficiency is common, people with COPD (particularly those below the age of 45 and with emphysema affecting the lower parts of the lungs) should be considered for testing.[15]
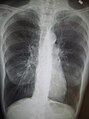
Chest X-ray demonstrating severe COPD: Note the small heart size in comparison to the lungs.
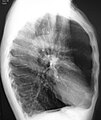
A lateral chest X-ray of a person with emphysema: Note the barrel chest and flat diaphragm.
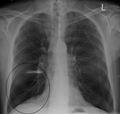
Lung bulla as seen on chest X-ray in a person with severe COPD

A severe case of bullous emphysema
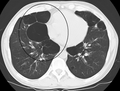
Axial CT image of the lung of a person with end-stage bullous emphysema
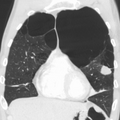
Very severe emphysema with lung cancer on the left (CT scan)
Differential diagnosis
COPD may need to be differentiated from other causes of shortness of breath such as congestive heart failure, pulmonary embolism, pneumonia, or pneumothorax. Many people with COPD mistakenly think they have asthma.[23] The distinction between asthma and COPD is made on the basis of the symptoms, smoking history, and whether airflow limitation is reversible with bronchodilators at spirometry.[69] Tuberculosis may also present with a chronic cough and should be considered in locations where it is common.[15] Less common conditions that may present similarly include bronchopulmonary dysplasia and obliterative bronchiolitis.[66] Chronic bronchitis may occur with normal airflow and in this situation it is not classified as COPD.[16]
Prevention
Most cases of COPD are potentially preventable through decreasing exposure to smoke and improving air quality.[11] Annual influenza vaccinations in those with COPD reduce exacerbations, hospitalizations and death.[70][71]Pneumococcal vaccination may also be beneficial.[70] The non-typable (unencapsulated) Haemophilus influenzae vaccine (NTHi) when taken by mouth does not appear to reduce severity or number of COPD exacerbations.[72] Eating a diet high in beta-carotene may help but taking supplements does not seem to.[73]
Smoking cessation
Keeping people from starting smoking is a key aspect of preventing COPD.[74] The policies of governments, public health agencies, and antismoking organizations can reduce smoking rates by discouraging people from starting and encouraging people to stop smoking.[75]Smoking bans in public areas and places of work are important measures to decrease exposure to secondhand smoke, and while many places have instituted bans, more are recommended.[11]
In those who smoke, stopping smoking is the only measure shown to slow down the worsening of COPD.[76][77] Even at a late stage of the disease, it can reduce the rate of worsening lung function and delay the onset of disability and death.[78] Often, several attempts are required before long-term abstinence is achieved.[75] Attempts over 5 years lead to success in nearly 40% of people.[79]
Some smokers can achieve long-term smoking cessation through willpower alone. Smoking, however, is highly addictive,[80] and many smokers need further support. The chance of quitting is improved with social support, engagement in a smoking cessation program, and the use of medications such as nicotine replacement therapy, bupropion, or varenicline.[75][77][79] Combining smoking-cessation medication with behavioral therapy is more than twice as likely to be effective in helping people with COPD stop smoking, compared with behavioral therapy alone.[81]
Occupational health
A number of measures have been taken to reduce the likelihood that workers in at-risk industries—such as coal mining, construction, and stonemasonry—will develop COPD.[11] Examples of these measures include the creation of public policy,[11] education of workers and management about the risks, promoting smoking cessation, checking workers for early signs of COPD, use of respirators, and dust control.[82][83] Effective dust control can be achieved by improving ventilation, using water sprays and by using mining techniques that minimize dust generation.[84] If a worker develops COPD, further lung damage can be reduced by avoiding ongoing dust exposure, for example by changing the work role.[85]
Air pollution
Both indoor and outdoor air quality can be improved, which may prevent COPD or slow the worsening of existing disease.[11] This may be achieved by public policy efforts, cultural changes, and personal involvement.[86]
A number of developed countries have successfully improved outdoor air quality through regulations. This has resulted in improvements in the lung function of their populations.[11] Those with COPD may experience fewer symptoms if they stay indoors on days when outdoor air quality is poor.[5]
One key effort is to reduce exposure to smoke from cooking and heating fuels through improved ventilation of homes and better stoves and chimneys.[86] Proper stoves may improve indoor air quality by 85%. Using alternative energy sources such as solar cooking and electrical heating is also effective. Using fuels such as kerosene or coal might be less bad than traditional biomass such as wood or dung.[11]
Management
No cure for COPD is known, but the symptoms are treatable and its progression can be delayed.[74] The major goals of management are to reduce risk factors, manage stable COPD, prevent and treat acute exacerbations, and manage associated illnesses.[5] The only measures that have been shown to reduce mortality are smoking cessation and supplemental oxygen.[87] Stopping smoking decreases the risk of death by 18%.[2] Other recommendations include influenza vaccination once a year, pneumococcal vaccination once every five years, and reduction in exposure to environmental air pollution.[2] In those with advanced disease, palliative care may reduce symptoms, with morphine improving the feelings of shortness of breath.[88]Noninvasive ventilation may be used to support breathing.[88] Providing people with a personalized action plan, an educational session, and support for use of their action plan in the event of an exacerbation, reduces the number of hospital visits and encourages early treatment of exacerbations.[89] When self-management interventions, such as taking corticosteroids and using supplemental oxygen, is combined with action plans, health-related quality of life is improved compared to usual care.[90]
Exercise
Pulmonary rehabilitation is a program of exercise, disease management, and counseling, coordinated to benefit the individual.[91] In those who have had a recent exacerbation, pulmonary rehabilitation appears to improve the overall quality of life and the ability to exercise.[92][93] If pulmonary rehabilitation improves mortality rates or hospital readmission rates is unclear.[92] Pulmonary rehabilitation has been shown to improve the sense of control a person has over their disease, as well as their emotions.[94]
The optimal exercise routine, use of noninvasive ventilation during exercise, and intensity of exercise suggested for people with COPD, is unknown.[93][95][96] Performing endurance arm exercises improves arm movement for people with COPD, and may result in a small improvement in breathlessness.[97] Performing arm exercises alone does not appear to improve quality of life.[97] Breathing exercises in and of themselves appear to have a limited role.[22]Pursed lip breathing exercises may be useful.[21][22]Tai chi exercises appear to be safe to practice for people with COPD, and may be beneficial for pulmonary function and pulmonary capacity when compared to a regular treatment program.[98] Tai Chi was not found to be more effective than other exercise intervention programs.[98] Inspiratory and expiratory muscle training (IMT, EMT) is an effective method for improving activities of daily living (ADL). A combination of IMT and walking exercises at home may help limit breathlessness in cases of severe COPD.[99] Additionally, the use of low amplitude high velocity joint mobilization together with exercise improves lung function and exercise capacity.[100] The goal of spinal manipulation therapy (SMT) is to improve thoracic mobility in an effort to reduce the work on the lungs during respiration, to in turn increase exercise capacity as indicated by the results of a systemic medical review.[100]
Being either underweight or overweight can affect the symptoms, degree of disability, and prognosis of COPD. People with COPD who are underweight can improve their breathing muscle strength by increasing their calorie intake.[5] When combined with regular exercise or a pulmonary rehabilitation program, this can lead to improvements in COPD symptoms. Supplemental nutrition may be useful in those who are malnourished.[101]
Bronchodilators
Inhaled bronchodilators are the primary medications used,[2] and result in a small overall benefit.[102] The two major types are β2 agonists and anticholinergics; both exist in long-acting and short-acting forms.[103] They reduce shortness of breath, wheeze, and exercise limitation, resulting in an improved quality of life.[104] It is unclear if they change the progression of the underlying disease.[2]
In those with mild disease, short-acting agents are recommended on an as needed basis.[2] In those with more severe disease, long-acting agents are recommended.[2] Long-acting agents partly work by reducing hyperinflation.[60] If long-acting bronchodilators are insufficient, then inhaled corticosteroids are typically added.[2] Which type of long-acting agent, tiotropium (a long-acting anticholinergic) or a long-acting beta agonist (LABA) is better is unclear, and trying each and continuing with the one that works best may be advisable.[105] Both types of agent appear to reduce the risk of acute exacerbations by 15–25%.[2] While both may be used at the same time, any added benefit is of questionable significance.[105]
Several short-acting β2 agonists are available, including salbutamol (albuterol) and terbutaline.[106] They provide some relief of symptoms for four to six hours.[106] LABAs such as salmeterol, formoterol, and indacaterol are often used as maintenance therapy. Some feel the evidence of benefits is limited,[107] while others view the evidence of benefit as established.[108][109][110] Long-term use appears safe in COPD[111] with adverse effects include shakiness and heart palpitations.[2] When used with inhaled steroids they increase the risk of pneumonia.[2] While steroids and LABAs may work better together,[107] it is unclear if this slight benefit outweighs the increased risks.[112] There is some evidence that combined treatment of LABAs with long-acting muscarinic antagonists (LAMA), an anticholinergic, may result in less exacerbations, less pneumonia, an improvement in forced expiratory volume (FEV1%), and potential improvements in quality of life when compared to treatment with LABA and an inhaled corticosteriod.[113] Indacaterol requires an inhaled dose once a day, and is as effective as the other long-acting β2 agonist drugs that require twice-daily dosing for people with stable COPD.[110]
Two main anticholinergics are used in COPD, ipratropium and tiotropium. Ipratropium is a short-acting agent, while tiotropium is long-acting. Tiotropium is associated with a decrease in exacerbations and improved quality of life,[114] and tiotropium provides those benefits better than ipratropium.[115] It does not appear to affect mortality or the overall hospitalization rate.[114] Anticholinergics can cause dry mouth and urinary tract symptoms.[2] They are also associated with increased risk of heart disease and stroke.[116][117]Aclidinium, another long-acting agent, reduces hospitalizations associated with COPD and improves quality of life.[118][119][120] The LAMA umeclidinium bromide is another anticholinergic alternative.[121] When compared to tiotropium, the LAMAs aclidinium, glycopyrronium, and umeclidinium appear to have a similar level of efficacy; with all four being more effective than placebo.[122] Further research is needed comparing aclidinium to tiotropium.[120]
Corticosteroids
Corticosteroids are usually used in inhaled form, but may also be used as tablets to treat and prevent acute exacerbations. While inhaled corticosteroids (ICSs) have not shown benefit for people with mild COPD, they decrease acute exacerbations in those with either moderate or severe disease.[123] By themselves, they have no effect on overall one-year mortality.[87][124] Whether they affect the progression of the disease is unknown.[2] When used in combination with a LABA, they may decrease mortality compared to either ICSs or LABA alone.[125][126] Inhaled steroids are associated with increased rates of pneumonia.[127] Long-term treatment with steroid tablets is associated with significant side effects.[106]
Other medication
Long-term antibiotics, specifically those from the macrolide class such as erythromycin, reduce the frequency of exacerbations in those who have two or more a year.[128][129] This practice may be cost effective in some areas of the world.[130] Concerns include that of antibiotic resistance and hearing problems with azithromycin.[129]Methylxanthines such as theophylline generally cause more harm than benefit and thus are usually not recommended,[131] but may be used as a second-line agent in those not controlled by other measures.[5]Mucolytics may help to reduce exacerbations in some people with chronic bronchitis.[132]Cough medicines are not recommended.[106]
For people with COPD, the use of cardioselective (heart-specific) beta-blocker therapy does not appear to impair respiratory function.[133] Cardioselective beta-blocker therapy should not be contraindicated for people with COPD.[133][134]
Oxygen
Supplemental oxygen is recommended in those with low oxygen levels at rest (a partial pressure of oxygen less than 50–55 mmHg or oxygen saturations of less than 88%).[106][135] In this group of people, it decreases the risk of heart failure and death if used 15 hours per day[106][135] and may improve people's ability to exercise.[136] In those with normal or mildly low oxygen levels, oxygen supplementation may improve shortness of breath when given during exercise, but may not improve breathlessness during normal daily activities or affect the quality of life.[137] A risk of fires and little benefit exist when those on oxygen continue to smoke.[138] In this situation, some recommend against its use.[139] During acute exacerbations, many require oxygen therapy; the use of high concentrations of oxygen without taking into account a person's oxygen saturations may lead to increased levels of carbon dioxide and worsened outcomes.[140][141] In those at high risk of high carbon dioxide levels, oxygen saturations of 88–92% are recommended, while for those without this risk, recommended levels are 94–98%.[141]
Surgery
For those with very severe disease, surgery is sometimes helpful and may include lung transplantation or lung volume-reduction surgery,[2] which involves removing the parts of the lung most damaged by emphysema, allowing the remaining, relatively good lung to expand and work better.[106] It seems to be particularly effective if emphysema predominantly involves the upper lobe, but the procedure increases the risks of early death and adverse events.[142] Lung transplantation is sometimes performed for very severe COPD, particularly in younger individuals.[106]
Exacerbations
Acute exacerbations are typically treated by increasing the use of short-acting bronchodilators.[2] This commonly includes a combination of a short-acting inhaled beta agonist and anticholinergic.[48] These medications can be given either via a metered-dose inhaler with a spacer or via a nebulizer, with both appearing to be equally effective.[48][143] Nebulization may be easier for those who are more unwell.[48]Oxygen supplementation can be useful. Excessive oxygen; however, can result in increased CO2 levels and a decreased level of consciousness.[144]
Corticosteroids by mouth improve the chance of recovery and decrease the overall duration of symptoms.[2][48] They work equally well as intravenous steroids but appear to have fewer side effects.[145] Five days of steroids work as well as ten or fourteen.[146] In those with a severe exacerbation, antibiotics improve outcomes.[147] A number of different antibiotics may be used including amoxicillin, doxycycline and azithromycin; whether one is better than the others is unclear.[70] The FDA recommends against the use of fluoroquinolones when other options are available due to higher risks of serious side effects.[148] There is no clear evidence for those with less severe cases.[147]
For those with type 2 respiratory failure (acutely raised CO2 levels) non-invasive positive pressure ventilation decreases the probability of death or the need of intensive care admission.[2] Additionally, theophylline may have a role in those who do not respond to other measures.[2] Fewer than 20% of exacerbations require hospital admission.[48] In those without acidosis from respiratory failure, home care ("hospital at home") may be able to help avoid some admissions.[48]
Prognosis

Chronic obstructive pulmonary disease deaths per million persons in 2012 .mw-parser-output .refbegin{font-size:90%;margin-bottom:0.5em}.mw-parser-output .refbegin-hanging-indents>ul{list-style-type:none;margin-left:0}.mw-parser-output .refbegin-hanging-indents>ul>li,.mw-parser-output .refbegin-hanging-indents>dl>dd{margin-left:0;padding-left:3.2em;text-indent:-3.2em;list-style:none}.mw-parser-output .refbegin-100{font-size:100%}
9–63
64–80
81–95
96–116
117–152
153–189
190–235
236–290
291–375
376–1089

Disability-adjusted life years lost to chronic obstructive pulmonary disease per 100,000 inhabitants in 2004.[149]
| no data ≤110 110–220 220–330 330–440 440–550 550–660 | 660–770 770–880 880–990 990–1100 1100–1350 ≥1350 |
COPD usually gets gradually worse over time and can ultimately result in death. It is estimated that 3% of all disability is related to COPD.[150] The proportion of disability from COPD globally has decreased from 1990 to 2010 due to improved indoor air quality primarily in Asia.[150] The overall number of years lived with disability from COPD, however, has increased.[151]
The rate at which COPD worsens varies with the presence of factors that predict a poor outcome, including severe airflow obstruction, little ability to exercise, shortness of breath, significant underweight or overweight, congestive heart failure, continued smoking, and frequent exacerbations.[5] Long-term outcomes in COPD can be estimated using the BODE index which gives a score of zero to ten depending on FEV1, body-mass index, the distance walked in six minutes, and the modified MRC dyspnea scale.[152] Significant weight loss is a bad sign.[16] Results of spirometry are also a good predictor of the future progress of the disease but not as good as the BODE index.[16][17]
Epidemiology
Globally, as of 2010, COPD affected approximately 329 million people (4.8% of the population).[151] The disease affects men and women almost equally, as there has been increased tobacco use among women in the developed world.[153] The increase in the developing world between 1970 and the 2000s is believed to be related to increasing rates of smoking in this region, an increasing population and an aging population due to fewer deaths from other causes such as infectious diseases.[2] Some developed countries have seen increased rates, some have remained stable and some have seen a decrease in COPD prevalence.[2] The global numbers are expected to continue increasing as risk factors remain common and the population continues to get older.[74]
Between 1990 and 2010 the number of deaths from COPD decreased slightly from 3.1 million to 2.9 million[154] and became the fourth leading cause of death.[2] In 2012 it became the third leading cause as the number of deaths rose again to 3.1 million.[155] In some countries, mortality has decreased in men but increased in women.[156] This is most likely due to rates of smoking in women and men becoming more similar.[16] COPD is more common in older people;[9] it affects 34–200 out of 1000 people older than 65 years, depending on the population under review.[9][67]
In England, an estimated 0.84 million people (of 50 million) have a diagnosis of COPD; this translates into approximately one person in 59 receiving a diagnosis of COPD at some point in their lives. In the most socioeconomically deprived parts of the country, one in 32 people were diagnosed with COPD, compared with one in 98 in the most affluent areas.[157] In the United States approximately 6.3% of the adult population, totaling approximately 15 million people, have been diagnosed with COPD.[158] 25 million people may have COPD if currently undiagnosed cases are included.[159] In 2011, there were approximately 730,000 hospitalizations in the United States for COPD.[160] In the United States, COPD is estimated to be the third leading cause of death in 2011.[161]
History

Giovanni Battista Morgagni, who made one of the earliest recorded descriptions of emphysema in 1769
The word "emphysema" is derived from the Greek ἐμφυσᾶν emphysan meaning "inflate" -itself composed of ἐν en, meaning "in", and φυσᾶν physan, meaning "breath, blast".[162] The term chronic bronchitis came into use in 1808[163] while the term COPD is believed to have first been used in 1965.[164] Previously it has been known by a number of different names, including chronic obstructive bronchopulmonary disease, chronic obstructive respiratory disease, chronic airflow obstruction, chronic airflow limitation, chronic obstructive lung disease, nonspecific chronic pulmonary disease, and diffuse obstructive pulmonary syndrome. The terms chronic bronchitis and emphysema were formally defined in 1959 at the CIBA guest symposium and in 1962 at the American Thoracic Society Committee meeting on Diagnostic Standards.[164]
Early descriptions of probable emphysema include: in 1679 by T. Bonet of a condition of "voluminous lungs" and in 1769 by Giovanni Morgagni of lungs which were "turgid particularly from air".[164][165] In 1721 the first drawings of emphysema were made by Ruysh.[165] These were followed with pictures by Matthew Baillie in 1789 and descriptions of the destructive nature of the condition. In 1814 Charles Badham used "catarrh" to describe the cough and excess mucus in chronic bronchitis. René Laennec, the physician who invented the stethoscope, used the term "emphysema" in his book A Treatise on the Diseases of the Chest and of Mediate Auscultation (1837) to describe lungs that did not collapse when he opened the chest during an autopsy. He noted that they did not collapse as usual because they were full of air and the airways were filled with mucus. In 1842, John Hutchinson invented the spirometer, which allowed the measurement of vital capacity of the lungs. However, his spirometer could measure only volume, not airflow. Tiffeneau and Pinelli in 1947 described the principles of measuring airflow.[164]
In 1953, Dr. George L. Waldbott, an American allergist, first described a new disease he named "smoker's respiratory syndrome" in the 1953 Journal of the American Medical Association. This was the first association between tobacco smoking and chronic respiratory disease.[166]
Early treatments included garlic, cinnamon and ipecac, among others.[163] Modern treatments were developed during the second half of the 20th century. Evidence supporting the use of steroids in COPD was published in the late 1950s. Bronchodilators came into use in the 1960s following a promising trial of isoprenaline. Further bronchodilators, such as salbutamol, were developed in the 1970s, and the use of LABAs began in the mid-1990s.[167]
Society and culture
COPD has been referred to as "smoker's lung".[168] People with emphysema have been known as "pink puffers" or "type A" due to their frequent pink complexion, fast respiratory rate and pursed lips,[169][170] and people with chronic bronchitis have been referred to as "blue bloaters" or "type B" due to the often bluish color of the skin and lips from low oxygen levels and their ankle swelling.[170][171] This terminology is no longer accepted as useful as most people with COPD have a combination of both emphysema and chronic bronchitis.[16][170]
Many health systems have difficulty ensuring appropriate identification, diagnosis and care of people with COPD; Britain's Department of Health has identified this as a major issue for the National Health Service and has introduced a specific strategy to tackle these problems.[172]
Economics
Globally, as of 2010, COPD is estimated to result in economic costs of $2.1 trillion, half of which occurring in the developing world.[14] Of this total an estimated $1.9 trillion are direct costs such as medical care, while $0.2 trillion are indirect costs such as missed work.[173] This is expected to more than double by the year 2030.[14] In Europe, COPD represents 3% of healthcare spending.[9] In the United States, costs of the disease are estimated at $50 billion, most of which is due to exacerbation.[9] COPD was among the most expensive conditions seen in U.S. hospitals in 2011, with a total cost of about $5.7 billion.[160]
Research
Infliximab, an immune-suppressing antibody, has been tested in COPD; there was a possibility of harm with no evidence of benefit.[174]
Roflumilast, cilomilast, and phosphodiesterase 4 inhibitors act as a bronchodilator and as an anti-inflammatory. They show promise in decreasing the rate of exacerbations, but do not appear to change a persons quality of life.[2][175] Roflumilast and cilomilast may be associated with side effects such as gastrointestinal issues and weight loss. Sleep disturbances and mood disturbances related to roflumilast have also been reported.[175] PDE4 is recommended to be used as an add-on therapy in case of failure of the standard COPD treatment during excerpations.[175]
Several new long-acting agents are under development.[2] Treatment with stem cells is under study.[176] While there is tentative data that it is safe, and the animal data is promising, there is little human data as of 2017.[177][178][179][180] The human data has shown poor results.[177][181]
A procedure known as targeted lung denervation, which involves decreasing the parasympathetic nervous system supply of the lungs, is being studied but does not have sufficient data to determine its use.[182] The effectiveness of alpha-1 antitrypsin augmentation treatment for people who have alpha-1 antitrypsin deficiency is unclear.[183]
Research continues into the use of telehealthcare to treat people with COPD when they experience episodes of shortness of breath; treating people remotely may reduce the number of emergency-room visits and improve the person's quality of life.[184]
Other animals
Chronic obstructive pulmonary disease may occur in a number of other animals and may be caused by exposure to tobacco smoke.[185][186] Most cases of the disease, however, are relatively mild.[187] In horses it is known as recurrent airway obstruction, can be quite severe, and most often is linked to an allergic reaction to a fungus contained in contaminated hay or straw.[188] COPD is also commonly found in old dogs.[189]
References
^ abcd Algusti AG, et al. (2017). "Definition and Overview". Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD). pp. 6–17..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ abcdefghijklmnopqrstuvwxyzaaabacadaeafagah Decramer M, Janssens W, Miravitlles M (April 2012). "Chronic obstructive pulmonary disease". Lancet. 379 (9823): 1341–51. doi:10.1016/S0140-6736(11)60968-9. PMID 22314182.
^ abcdefghijklm "Chronic obstructive pulmonary disease (COPD) Fact sheet N°315". WHO. January 2015. Archived from the original on 4 March 2016. Retrieved 4 March 2016.
^ ab Nathell L, Nathell M, Malmberg P, Larsson K (December 2007). "COPD diagnosis related to different guidelines and spirometry techniques". Respiratory Research. 8 (1): 89. doi:10.1186/1465-9921-8-89. PMC 2217523. PMID 18053200.
^ abcdefghijkl Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J (September 2007). "Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary". American Journal of Respiratory and Critical Care Medicine. 176 (6): 532–55. doi:10.1164/rccm.200703-456SO. PMID 17507545.
^ ab GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
^ ab GBD 2015 Mortality and Causes of Death Collaborators (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
^ Roversi S, Corbetta L, Clini E (5 May 2017). "GOLD 2017 recommendations for COPD patients: toward a more personalized approach". COPD Research and Practice. 3. doi:10.1186/s40749-017-0024-y.
^ abcdefghijklmnopqrstuvwxyzaa Vestbo J (2013). "Definition and Overview". Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease. pp. 1–7.
^ Craig JA (2012). Ferri's netter patient advisor (2nd ed.). Saunders. p. 913. ISBN 9781455728268.
^ abcdefghijklmn Pirozzi C, Scholand MB (July 2012). "Smoking cessation and environmental hygiene". The Medical Clinics of North America. 96 (4): 849–67. doi:10.1016/j.mcna.2012.04.014. PMID 22793948.
^ GBD 2013 Mortality and Causes of Death Collaborators (January 2015). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013". Lancet. 385 (9963): 117–71. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604. PMID 25530442.
^ Mathers CD, Loncar D (November 2006). "Projections of global mortality and burden of disease from 2002 to 2030". PLoS Medicine. 3 (11): e442. doi:10.1371/journal.pmed.0030442. PMC 1664601. PMID 17132052.
^ abc Lomborg B (2013). Global problems, local solutions : costs and benefits. Cambridge University Press. p. 143. ISBN 978-1-107-03959-9.
^ abcdefghijklmnopqrstu Vestbo J (2013). "Diagnosis and Assessment" (PDF). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease. pp. 9–17.
[dead link]
^ abcdefghijklm Reilly JJ, Silverman EK, Shapiro SD (2011). "Chronic Obstructive Pulmonary Disease". In Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J. Harrison's Principles of Internal Medicine (18th ed.). McGraw Hill. pp. 2151–9. ISBN 978-0-07-174889-6.
^ abcdefg National Institute for Health and Clinical Excellence. Clinical guideline 101: Chronic Obstructive Pulmonary Disease. London, June 2010.
^ Mahler DA (May 2006). "Mechanisms and measurement of dyspnea in chronic obstructive pulmonary disease". Proceedings of the American Thoracic Society. 3 (3): 234–8. doi:10.1513/pats.200509-103SF. PMID 16636091.
^ "What Are the Signs and Symptoms of COPD?". National Heart, Lung, and Blood Institute. July 31, 2013. Archived from the original on November 18, 2013. Retrieved November 29, 2013.
^ MedlinePlus Encyclopedia Chronic obstructive pulmonary disease
^ ab Goldstein NE, Morrison RS (2013). Evidence-based practice of palliative medicine. Elsevier/Saunders. p. 124. ISBN 978-1-4377-3796-7.
^ abc Holland AE, Hill CJ, Jones AY, McDonald CF (October 2012). Holland AE, ed. "Breathing exercises for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 10: CD008250. doi:10.1002/14651858.CD008250.pub2. PMID 23076942.
^ abcdef Gruber P (November 2008). "The Acute Presentation of Chronic Obstructive Pulmonary Disease In the Emergency Department: A Challenging Oxymoron". Emergency Medicine Practice. 10 (11). Archived from the original on 2013-10-05.
^ ab Weitzenblum E, Chaouat A (2009). "Cor pulmonale". Chronic Respiratory Disease. 6 (3): 177–85. doi:10.1177/1479972309104664. PMID 19643833.
^ "Cor pulmonale". Professional guide to diseases (9th ed.). Wolters Kluwer Health/Lippincott Williams & Wilkins. 2009. pp. 120–2. ISBN 978-0-7817-7899-2.
^ Levack WM, Poot B, Weatherall M, Travers J, Levack WM (2015). "Interventions for sexual dysfunction in people with chronic obstructive pulmonary disease (COPD)". Reviews. doi:10.1002/14651858.CD011442.pub2.
^ Aboussouan L (2009). "Chapter 35: Obstructive Lung Diseae: Asthma and Chronic Obstructive Pulmonary Disease". In Stoller JK, Michota FA, Mandell BF. The Cleveland Clinic Foundation intensive review of internal medicine (5th ed.). Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 419. ISBN 978-0-7817-9079-6.
^ Brulotte CA, Lang ES (May 2012). "Acute exacerbations of chronic obstructive pulmonary disease in the emergency department". Emergency Medicine Clinics of North America. 30 (2): 223–47, vii. doi:10.1016/j.emc.2011.10.005. PMID 22487106.
^ Spiro S (2012). "Chapter 43: Management of Exacerbations in Chronic Obstructive Pulmonary Disease". Clinical respiratory medicine expert consult (4th ed.). Saunders. ISBN 978-1-4557-2329-4.
^ World Health Organization (2008). WHO Report on the Global Tobacco Epidemic 2008: The MPOWER Package (PDF). World Health Organization. pp. 268–309. ISBN 92-4-159628-7. Archived (PDF) from the original on 2013-11-12.
^ ab Ward H (2012). Oxford Handbook of Epidemiology for Clinicians. Oxford University Press. pp. 289–290. ISBN 978-0-19-165478-7.
^ Laniado-Laborín R (January 2009). "Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century". International Journal of Environmental Research and Public Health. 6 (1): 209–24. doi:10.3390/ijerph6010209. PMC 2672326. PMID 19440278.
^ ab Rennard S (2013). Clinical management of chronic obstructive pulmonary disease (2nd ed.). Informa Healthcare. p. 23. ISBN 978-0-8493-7588-0.
^ ab Sharma A, Barclay J (2010). COPD in primary care. Radcliffe Pub. p. 9. ISBN 978-1-84619-316-3.
^ Goldman L (2012). Goldman's Cecil medicine (24th ed.). Elsevier/Saunders. p. 537. ISBN 978-1-4377-1604-7.
^ Raad D, Gaddam S, Schunemann HJ, Irani J, Abou Jaoude P, Honeine R, Akl EA (April 2011). "Effects of water-pipe smoking on lung function: a systematic review and meta-analysis". Chest. 139 (4): 764–774. doi:10.1378/chest.10-0991. PMID 20671057.
^ Joshi M, Joshi A, Bartter T (March 2014). "Marijuana and lung diseases". Current Opinion in Pulmonary Medicine. 20 (2): 173–9. doi:10.1097/MCP.0000000000000026. PMID 24384575.
^ Amaral AF, Strachan DP, Burney PG, Jarvis DL (May 2017). "Female Smokers Are at Greater Risk of Airflow Obstruction Than Male Smokers. UK Biobank" (PDF). American Journal of Respiratory and Critical Care Medicine. 195 (9): 1226–1235. doi:10.1164/rccm.201608-1545OC. PMID 28075609.
^ ab Kennedy SM, Chambers R, Du W, Dimich-Ward H (December 2007). "Environmental and occupational exposures: do they affect chronic obstructive pulmonary disease differently in women and men?". Proceedings of the American Thoracic Society. 4 (8): 692–4. doi:10.1513/pats.200707-094SD. PMID 18073405.
^ Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM (September 2006). "Global burden of COPD: systematic review and meta-analysis". The European Respiratory Journal. 28 (3): 523–32. doi:10.1183/09031936.06.00124605. PMID 16611654.
^ ab Devereux G (May 2006). "ABC of chronic obstructive pulmonary disease. Definition, epidemiology, and risk factors". BMJ. 332 (7550): 1142–4. doi:10.1136/bmj.332.7550.1142. PMC 1459603. PMID 16690673.
^ Laine C (2009). In the Clinic: Practical Information about Common Health Problems. ACP Press. p. 226. ISBN 978-1-934465-64-6.
^ ab Barnes PJ, Drazen JM, Rennard SI, Thomson NC, eds. (2009). "Relationship between cigarette smoking and occupational exposures". Asthma and COPD: Basic Mechanisms and Clinical Management. Academic. p. 464. ISBN 978-0-12-374001-4.
^ Rushton L (2007). "Chronic obstructive pulmonary disease and occupational exposure to silica". Reviews on Environmental Health. 22 (4): 255–72. doi:10.1515/REVEH.2007.22.4.255. PMID 18351226.
^ Hopper T (2014). Mosby's Pharmacy Technician – E-Book: Principles and Practice. Elsevier Health Sciences. p. 610. ISBN 9780323292450.
^ abcd Foreman MG, Campos M, Celedón JC (July 2012). "Genes and chronic obstructive pulmonary disease". The Medical Clinics of North America. 96 (4): 699–711. doi:10.1016/j.mcna.2012.02.006. PMC 3399759. PMID 22793939.
^ Brode SK, Ling SC, Chapman KR (September 2012). "Alpha-1 antitrypsin deficiency: a commonly overlooked cause of lung disease". CMAJ. 184 (12): 1365–71. doi:10.1503/cmaj.111749. PMC 3447047. PMID 22761482.
^ abcdefg Vestbo J (2013). "Management of Exacerbations" (PDF). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease. pp. 39–45.
[dead link]
^ abc Dhar R (2011). Textbook of pulmonary and critical care medicine. New Delhi: Jaypee Brothers Medical Publishers. p. 1056. ISBN 978-93-5025-073-0.
^ Palange P (2013). ERS Handbook of Respiratory Medicine. European Respiratory Society. p. 194. ISBN 978-1-84984-041-5.
^ Lötvall J (2011). "Anti-infective treatments in asthma and COPD (10)". Advances in combination therapy for asthma and COPD. Wiley. p. 251. ISBN 978-1-119-97846-6.
^ Barnes P (2009). Asthma and COPD : basic mechanisms and clinical management (2nd ed.). Academic. p. 837. ISBN 978-0-12-374001-4.
^ Hanania N (2010-12-09). COPD a Guide to Diagnosis and Clinical Management (1st ed.). Springer Science+Business Media, LLC. p. 197. ISBN 978-1-59745-357-8.
^ ab Beasley V, Joshi PV, Singanayagam A, Molyneaux PL, Johnston SL, Mallia P (2012). "Lung microbiology and exacerbations in COPD". International Journal of Chronic Obstructive Pulmonary Disease. 7: 555–69. doi:10.2147/COPD.S28286. PMC 3437812. PMID 22969296.
^ Aleva FE, Voets LW, Simons SO, de Mast Q, van der Ven AJ, Heijdra YF (March 2017). "Prevalence and Localization of Pulmonary Embolism in Unexplained Acute Exacerbations of COPD: A Systematic Review and Meta-analysis". Chest. 151 (3): 544–554. doi:10.1016/j.chest.2016.07.034. PMID 27522956.
^ Murphy DM, Fishman AP (2008). "Chapter 53". Fishman's Pulmonary Diseases and Disorders (4th ed.). McGraw-Hill. p. 913. ISBN 0-07-145739-9.
^ ab Calverley PM, Koulouris NG (January 2005). "Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology". The European Respiratory Journal. 25 (1): 186–99. doi:10.1183/09031936.04.00113204. PMID 15640341.
^ Currie GP (2010). ABC of COPD (2nd ed.). Wiley-Blackwell, BMJ Books. p. 32. ISBN 978-1-4443-2948-3.
^ O'Donnell DE (April 2006). "Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease". Proceedings of the American Thoracic Society. 3 (2): 180–4. doi:10.1513/pats.200508-093DO. PMID 16565429.
^ ab Cooper CB (October 2006). "The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function". The American Journal of Medicine. 119 (10 Suppl 1): 21–31. doi:10.1016/j.amjmed.2006.08.004. PMID 16996896.
^ abcdef Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schünemann H, Wedzicha W, MacDonald R, Shekelle P (August 2011). "Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society". Annals of Internal Medicine. 155 (3): 179–91. doi:10.7326/0003-4819-155-3-201108020-00008. PMID 21810710. Archived from the original on 2014-10-12.
^ Siu AL, Bibbins-Domingo K, Grossman DC, Davidson KW, Epling JW, García FA, Gillman M, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Harper DM, Phillips WR, Phipps MG, Pignone MP (April 2016). "Screening for Chronic Obstructive Pulmonary Disease: US Preventive Services Task Force Recommendation Statement". JAMA. 315 (13): 1372–7. doi:10.1001/jama.2016.2638. PMID 27046365.
^ ab Young VB (2010). Blueprints medicine (5th ed.). Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 69. ISBN 978-0-7817-8870-0.
^ Bailey KL (July 2012). "The importance of the assessment of pulmonary function in COPD". The Medical Clinics of North America. 96 (4): 745–52. doi:10.1016/j.mcna.2012.04.011. PMC 3998207. PMID 22793942.
^ "COPD Assessment Test (CAT)". American Thoracic Society. Archived from the original on December 3, 2013. Retrieved November 29, 2013.
^ ab National Institute for Health and Clinical Excellence. Clinical guideline 101: Chronic Obstructive Pulmonary Disease. London, June 2010.
^ ab Torres M, Moayedi S (May 2007). "Evaluation of the acutely dyspneic elderly patient". Clinics in Geriatric Medicine. 23 (2): 307–25, vi. doi:10.1016/j.cger.2007.01.007. PMID 17462519.
^ Brant, William E.; Helms, Clyde A. (2007). Fundamentals of Diagnostic Radiology. Lippincott Williams & Wilkins. p. 513. ISBN 9780781761352.
^ BTS COPD Consortium (2005). "Spirometry in practice – a practical guide to using spirometry in primary care". pp. 8–9. Archived from the original on 26 August 2014. Retrieved 25 August 2014.
^ abc Mackay AJ, Hurst JR (July 2012). "COPD exacerbations: causes, prevention, and treatment". The Medical Clinics of North America. 96 (4): 789–809. doi:10.1016/j.mcna.2012.02.008. PMID 22793945.
^ Kopsaftis, Z; Wood-Baker, R; Poole, P (26 June 2018). "Influenza vaccine for chronic obstructive pulmonary disease (COPD)". The Cochrane Database of Systematic Reviews. 6: CD002733. doi:10.1002/14651858.CD002733.pub3. PMID 29943802.
^ Teo, E; Lockhart, K; Purchuri, SN; Pushparajah, J; Cripps, AW; van Driel, ML (19 June 2017). "Haemophilus influenzae oral vaccination for preventing acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 6: CD010010. doi:10.1002/14651858.CD010010.pub3. PMID 28626902.
^ "Beta-carotene: MedlinePlus Supplements". medlineplus.gov. Archived from the original on 26 December 2016. Retrieved 26 December 2016.
^ abc Vestbo J (2013). "Introduction". Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (PDF). Global Initiative for Chronic Obstructive Lung Disease. xiii–xv. Archived from the original (PDF) on 2013-10-04.
^ abc Policy Recommendations for Smoking Cessation and Treatment of Tobacco Dependence. World Health Organization. pp. 15–40. ISBN 978-92-4-156240-9. Archived from the original on 2008-09-15.
^ Jiménez-Ruiz CA, Fagerström KO (March 2013). "Smoking cessation treatment for COPD smokers: the role of counselling". Monaldi Archives for Chest Disease = Archivio Monaldi Per Le Malattie Del Torace. 79 (1): 33–7. doi:10.4081/monaldi.2013.107. PMID 23741944.
^ ab "Chronic obstructive pulmonary disease in over 16s: diagnosis and management | Guidance and guidelines | NICE". www.nice.org.uk. Retrieved 2018-06-05.
^ Kumar P, Clark M (2005). Clinical Medicine (6th ed.). Elsevier Saunders. pp. 900–1. ISBN 0-7020-2763-4.
^ ab Tønnesen P (March 2013). "Smoking cessation and COPD". European Respiratory Review. 22 (127): 37–43. doi:10.1183/09059180.00007212. PMID 23457163.
^ "Why is smoking addictive?". NHS Choices. December 29, 2011. Archived from the original on October 13, 2013. Retrieved November 29, 2013.
^ van Eerd EA, van der Meer RM, van Schayck OC, Kotz D (August 2016). "Smoking cessation for people with chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews (8): CD010744. doi:10.1002/14651858.CD010744.pub2. PMID 27545342.
^ Smith BK, Timby NE (2005). Essentials of nursing : care of adults and children. Lippincott Williams & Wilkins. p. 338. ISBN 978-0-7817-5098-1.
^ Rom WN, Markowitz SB, eds. (2007). Environmental and occupational medicine (4th ed.). Wolters Kluwer/Lippincott Williams & Wilkins. pp. 521–2. ISBN 978-0-7817-6299-1.
^ "Wet cutting". Health and Safety Executive. Archived from the original on December 3, 2013. Retrieved November 29, 2013.
^ George RB (2005). Chest medicine : essentials of pulmonary and critical care medicine (5th ed.). Lippincott Williams & Wilkins. p. 172. ISBN 978-0-7817-5273-2.
^ ab Vestbo J (2013). "Management of Stable COPD". Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (PDF). Global Initiative for Chronic Obstructive Lung Disease. pp. 31–8. Archived from the original (PDF) on 2013-10-04.
^ ab Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E (November 2008). "Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis". JAMA. 300 (20): 2407–16. doi:10.1001/jama.2008.717. PMC 4804462. PMID 19033591.
^ ab Carlucci A, Guerrieri A, Nava S (December 2012). "Palliative care in COPD patients: is it only an end-of-life issue?". European Respiratory Review. 21 (126): 347–54. doi:10.1183/09059180.00001512. PMID 23204123.
^ Howcroft M, Walters EH, Wood-Baker R, Walters JA (December 2016). "Action plans with brief patient education for exacerbations in chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 12: CD005074. doi:10.1002/14651858.CD005074.pub4. PMID 27990628.
^ Lenferink A, Brusse-Keizer M, van der Valk PD, Frith PA, Zwerink M, Monninkhof EM, van der Palen J, Effing TW (August 2017). "Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 8: CD011682. doi:10.1002/14651858.CD011682.pub2. PMID 28777450.
^ "COPD — Treatment". U.S. National Heart Lung and Blood Institute. Archived from the original on 2012-04-27. Retrieved 2013-07-23.
^ ab Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T (December 2016). "Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 12: CD005305. doi:10.1002/14651858.CD005305.pub4. PMID 27930803.
^ ab Zainuldin R, Mackey MG, Alison JA (November 2011). "Optimal intensity and type of leg exercise training for people with chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews (11): CD008008. doi:10.1002/14651858.CD008008.pub2. PMID 22071841.
^ McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y (February 2015). "Pulmonary rehabilitation for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 2 (2): CD003793. doi:10.1002/14651858.CD003793.pub3. PMID 25705944.
^ McNamara RJ, McKeough ZJ, McKenzie DK, Alison JA (December 2013). "Water-based exercise training for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews (12): CD008290. doi:10.1002/14651858.CD008290.pub2. PMID 24353107.
^ Menadue C, Piper AJ, van 't Hul AJ, Wong KK (May 2014). "Non-invasive ventilation during exercise training for people with chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews (5): CD007714. doi:10.1002/14651858.CD007714.pub2. PMID 24823712.
^ ab McKeough ZJ, Velloso M, Lima VP, Alison JA (November 2016). "Upper limb exercise training for COPD". The Cochrane Database of Systematic Reviews. 11: CD011434. doi:10.1002/14651858.CD011434.pub2. PMID 27846347.
^ ab Ngai SP, Jones AY, Tam WW (June 2016). "Tai Chi for chronic obstructive pulmonary disease (COPD)". The Cochrane Database of Systematic Reviews (6): CD009953. doi:10.1002/14651858.CD009953.pub2. PMID 27272131.
^ Thomas MJ, Simpson J, Riley R, Grant E (June 2010). "The impact of home-based physiotherapy interventions on breathlessness during activities of daily living in severe COPD: a systematic review". Physiotherapy. 96 (2): 108–19. doi:10.1016/j.physio.2009.09.006. PMID 20420957.
^ ab Wearing J, Beaumont S, Forbes D, Brown B, Engel R (February 2016). "The Use of Spinal Manipulative Therapy in the Management of Chronic Obstructive Pulmonary Disease: A Systematic Review". Journal of Alternative and Complementary Medicine. 22 (2): 108–14. doi:10.1089/acm.2015.0199. PMID 26700633.
^ Ferreira IM, Brooks D, White J, Goldstein R (December 2012). Ferreira IM, ed. "Nutritional supplementation for stable chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 12: CD000998. doi:10.1002/14651858.CD000998.pub3. PMID 23235577.
^ van Dijk WD, van den Bemt L, van Weel C (2013). "Megatrials for bronchodilators in chronic obstructive pulmonary disease (COPD) treatment: time to reflect". Journal of the American Board of Family Medicine. 26 (2): 221–4. doi:10.3122/jabfm.2013.02.110342. PMID 23471939.
^ Kew KM, Dias S, Cates CJ (March 2014). "Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis". The Cochrane Database of Systematic Reviews (3): CD010844. doi:10.1002/14651858.CD010844.pub2. PMID 24671923.
^ Liesker JJ, Wijkstra PJ, Ten Hacken NH, Koëter GH, Postma DS, Kerstjens HA (February 2002). "A systematic review of the effects of bronchodilators on exercise capacity in patients with COPD". Chest. 121 (2): 597–608. doi:10.1378/chest.121.2.597. PMID 11834677.
^ ab Farne HA, Cates CJ (October 2015). "Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews (10): CD008989. doi:10.1002/14651858.CD008989.pub3. PMID 26490945.
^ abcdefgh Vestbo J (2013). "Therapeutic Options" (PDF). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease. pp. 19–30.
[dead link]
^ ab Cave AC, Hurst MM (May 2011). "The use of long acting β₂-agonists, alone or in combination with inhaled corticosteroids, in chronic obstructive pulmonary disease (COPD): a risk-benefit analysis". Pharmacology & Therapeutics. 130 (2): 114–43. doi:10.1016/j.pharmthera.2010.12.008. PMID 21276815.
^ Spencer S, Karner C, Cates CJ, Evans DJ (December 2011). Spencer S, ed. "Inhaled corticosteroids versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews (12): CD007033. doi:10.1002/14651858.CD007033.pub3. PMID 22161409.
^ Wang J, Nie B, Xiong W, Xu Y (April 2012). "Effect of long-acting beta-agonists on the frequency of COPD exacerbations: a meta-analysis". Journal of Clinical Pharmacy and Therapeutics. 37 (2): 204–11. doi:10.1111/j.1365-2710.2011.01285.x. PMID 21740451.
^ ab Geake JB, Dabscheck EJ, Wood-Baker R, Cates CJ (January 2015). "Indacaterol, a once-daily beta2-agonist, versus twice-daily beta₂-agonists or placebo for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 1: CD010139. doi:10.1002/14651858.CD010139.pub2. PMID 25575340.
^ Decramer ML, Hanania NA, Lötvall JO, Yawn BP (2013). "The safety of long-acting β2-agonists in the treatment of stable chronic obstructive pulmonary disease". International Journal of Chronic Obstructive Pulmonary Disease. 8: 53–64. doi:10.2147/COPD.S39018. PMC 3558319. PMID 23378756.
^ Nannini LJ, Lasserson TJ, Poole P (September 2012). Nannini LJ, ed. "Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 9 (9): CD006829. doi:10.1002/14651858.CD006829.pub2. PMC 4170910. PMID 22972099.
^ Horita N, Goto A, Shibata Y, Ota E, Nakashima K, Nagai K, Kaneko T (February 2017). "Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD)". The Cochrane Database of Systematic Reviews. 2: CD012066. doi:10.1002/14651858.CD012066.pub2. PMID 28185242.
^ ab Karner C, Chong J, Poole P (July 2014). "Tiotropium versus placebo for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews (7): CD009285. doi:10.1002/14651858.CD009285.pub3. PMID 25046211.
^ Cheyne L, Irvin-Sellers MJ, White J (September 2015). "Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews (9): CD009552. doi:10.1002/14651858.CD009552.pub3. PMID 26391969.
^ Singh S, Loke YK, Furberg CD (September 2008). "Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis". JAMA. 300 (12): 1439–50. doi:10.1001/jama.300.12.1439. PMID 18812535.
^ Singh S, Loke YK, Enright P, Furberg CD (January 2013). "Pro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medications". Thorax. 68 (1): 114–6. doi:10.1136/thoraxjnl-2011-201275. PMID 22764216.
^ Jones P (April 2013). "Aclidinium bromide twice daily for the treatment of chronic obstructive pulmonary disease: a review". Advances in Therapy. 30 (4): 354–68. doi:10.1007/s12325-013-0019-2. PMID 23553509.
^ Cazzola M, Page CP, Matera MG (June 2013). "Aclidinium bromide for the treatment of chronic obstructive pulmonary disease". Expert Opinion on Pharmacotherapy. 14 (9): 1205–14. doi:10.1517/14656566.2013.789021. PMID 23566013.
^ ab Ni H, Soe Z, Moe S (September 2014). "Aclidinium bromide for stable chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 9 (9): CD010509. doi:10.1002/14651858.CD010509.pub2. PMID 25234126.
^ Ni H, Htet A, Moe S (June 2017). "Umeclidinium bromide versus placebo for people with chronic obstructive pulmonary disease (COPD)". The Cochrane Database of Systematic Reviews. 6: CD011897. doi:10.1002/14651858.CD011897.pub2. PMID 28631387.
^ Ismaila AS, Huisman EL, Punekar YS, Karabis A (2015). "Comparative efficacy of long-acting muscarinic antagonist monotherapies in COPD: a systematic review and network meta-analysis". International Journal of Chronic Obstructive Pulmonary Disease. 10: 2495–517. doi:10.2147/COPD.S92412. PMC 4655912. PMID 26604738.
^ Gartlehner G, Hansen RA, Carson SS, Lohr KN (2006). "Efficacy and safety of inhaled corticosteroids in patients with COPD: a systematic review and meta-analysis of health outcomes". Annals of Family Medicine. 4 (3): 253–62. doi:10.1370/afm.517. PMC 1479432. PMID 16735528.
^ Chinet T, Dumoulin J, Honore I, Braun JM, Couderc LJ, Febvre M, Mangiapan G, Maurer C, Serrier P, Soyez F, Terrioux P, Jebrak G (December 2016). "[The place of inhaled corticosteroids in COPD]". Revue Des Maladies Respiratoires. 33 (10): 877–891. doi:10.1016/j.rmr.2015.11.009. PMID 26831345.
^ Dong YH, Lin HH, Shau WY, Wu YC, Chang CH, Lai MS (January 2013). "Comparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta-analysis of randomised controlled trials". Thorax. 68 (1): 48–56. doi:10.1136/thoraxjnl-2012-201926. PMID 23042705.
^ Nannini LJ, Poole P, Milan SJ, Kesterton A (August 2013). "Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 8 (8): CD006826. doi:10.1002/14651858.CD006826.pub2. PMID 23990350.
^ Kew KM, Seniukovich A (March 2014). "Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 3 (3): CD010115. doi:10.1002/14651858.CD010115.pub2. PMID 24615270.
^ Mammen MJ, Sethi S (2012). "Macrolide therapy for the prevention of acute exacerbations in chronic obstructive pulmonary disease". Polskie Archiwum Medycyny Wewnetrznej. 122 (1–2): 54–9. PMID 22353707.
^ ab Herath SC, Poole P (November 2013). "Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD)". The Cochrane Database of Systematic Reviews. 11 (11): CD009764. doi:10.1002/14651858.CD009764.pub2. PMID 24288145.
^ Simoens S, Laekeman G, Decramer M (May 2013). "Preventing COPD exacerbations with macrolides: a review and budget impact analysis". Respiratory Medicine. 107 (5): 637–48. doi:10.1016/j.rmed.2012.12.019. PMID 23352223.
^ Barr RG, Rowe BH, Camargo CA (2003). Barr RG, ed. "Methylxanthines for exacerbations of chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews (2): CD002168. doi:10.1002/14651858.CD002168. PMID 12804425.
^ Poole P, Chong J, Cates CJ (July 2015). "Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 7 (7): CD001287. doi:10.1002/14651858.CD001287.pub5. PMID 26222376.
^ ab Salpeter S, Ormiston T, Salpeter E (October 2005). "Cardioselective beta-blockers for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews (4): CD003566. doi:10.1002/14651858.CD003566.pub2. PMID 16235327.
^ Ni Y, Shi G, Wan H (2012). "Use of cardioselective β-blockers in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized, placebo-controlled, blinded trials". The Journal of International Medical Research. 40 (6): 2051–65. doi:10.1177/030006051204000602. PMID 23321161.
^ ab COPD Working Group (2012). "Long-term oxygen therapy for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis". Ontario Health Technology Assessment Series. 12 (7): 1–64. PMC 3384376. PMID 23074435.
^ Bradley JM, O'Neill B (October 2005). Bradley JM, ed. "Short-term ambulatory oxygen for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 4 (4): CD004356. doi:10.1002/14651858.CD004356.pub3. PMID 16235359.
^ Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D (November 2016). "Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy". The Cochrane Database of Systematic Reviews. 11: CD006429. doi:10.1002/14651858.CD006429.pub3. PMID 27886372.
^ Chapman S (2009). Oxford handbook of respiratory medicine (2nd ed.). Oxford University Press. p. 707. ISBN 978-0-19-954516-2.
^ Blackler L (2007). Managing chronic obstructive pulmonary disease. Wiley. p. 49. ISBN 978-0-470-51798-7.
^ Jindal SK (2013). Chronic Obstructive Pulmonary Disease. Jaypee Brothers Medical. p. 139. ISBN 978-93-5090-353-7.
^ ab O'Driscoll BR, Howard LS, Davison AG (October 2008). "BTS guideline for emergency oxygen use in adult patients". Thorax. 63 Suppl 6 (Suppl 6): vi1–68. doi:10.1136/thx.2008.102947. PMID 18838559.
^ van Agteren JE, Carson KV, Tiong LU, Smith BJ (October 2016). "Lung volume reduction surgery for diffuse emphysema". The Cochrane Database of Systematic Reviews. 10: CD001001. doi:10.1002/14651858.CD001001.pub3. PMID 27739074.
^ "Bronchodilators delivered by nebuliser versus inhalers for lung attacks of chronic obstructive pulmonary disease". Cochrane Database of Systematic Reviews. 29 August 2016. doi:10.1002/14651858.cd011826. Archived from the original on 13 September 2016.
^ Wise R. "Chronic Obstructive Pulmonary Disease (COPD) – Pulmonary Disorders – Merck Manuals Professional Edition". Merck Manuals Professional Edition. Archived from the original on 28 December 2016. Retrieved 16 December 2016.
^ Walters JA, Tan DJ, White CJ, Gibson PG, Wood-Baker R, Walters EH (September 2014). "Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 9 (9): CD001288. doi:10.1002/14651858.CD001288.pub4. PMID 25178099. Archived from the original on 2014-09-24.
^ Walters JA, Tan DJ, White CJ, Wood-Baker R (March 2018). "Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 3: CD006897. doi:10.1002/14651858.CD006897.pub4. PMID 29553157.
^ ab Vollenweider DJ, Jarrett H, Steurer-Stey CA, Garcia-Aymerich J, Puhan MA (December 2012). Vollenweider DJ, ed. "Antibiotics for exacerbations of chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 12: CD010257. doi:10.1002/14651858.CD010257. PMID 23235687.
^ "Fluoroquinolone Antibacterial Drugs: Drug Safety Communication – FDA Advises Restricting Use for Certain Uncomplicated Infections". FDA. 12 May 2016. Archived from the original on 16 May 2016. Retrieved 16 May 2016.
^ "WHO Disease and injury country estimates". World Health Organization. 2009. Archived from the original on 2009-11-11. Retrieved Nov 11, 2009.
^ ab Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. (December 2012). "Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2197–223. doi:10.1016/S0140-6736(12)61689-4. PMID 23245608.
^ ab Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. (December 2012). "Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2163–96. doi:10.1016/S0140-6736(12)61729-2. PMID 23245607.
^ prepared by the Department of Medicine, Washington University School of Medicine (2009). The Washington manual general internal medicine subspecialty consult (2nd ed.). Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 96. ISBN 978-0-7817-9155-7.
^ "Chronic obstructive pulmonary disease (COPD) Fact sheet N°315". WHO. January 2015. Archived from the original on 2016-03-04.
^ Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. (December 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604.
^ "The 10 leading causes of death in the world, 2000 and 2011". World Health Organization. July 2013. Archived from the original on December 2, 2013. Retrieved November 29, 2013.
^ Rycroft CE, Heyes A, Lanza L, Becker K (2012). "Epidemiology of chronic obstructive pulmonary disease: a literature review". International Journal of Chronic Obstructive Pulmonary Disease. 7: 457–94. doi:10.2147/COPD.S32330. PMC 3422122. PMID 22927753.
^ Simpson CR, Hippisley-Cox J, Sheikh A (July 2010). "Trends in the epidemiology of chronic obstructive pulmonary disease in England: a national study of 51 804 patients". The British Journal of General Practice. 60 (576): 277–84. doi:10.3399/bjgp10X514729. PMC 2894402. PMID 20594429.
^ Centers for Disease Control and Prevention (November 2012). "Chronic obstructive pulmonary disease among adults--United States, 2011". MMWR. Morbidity and Mortality Weekly Report. 61 (46): 938–43. PMID 23169314. Archived from the original on 3 December 2013.
^ "Morbidity & Mortality: 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases" (PDF). National Heart, Lung, and Blood Institute. Archived from the original (PDF) on 2013-10-19.
^ ab Torio CM, Andrews RM (2006). "National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011: Statistical Brief #160". Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Health Care Policy and Research. PMID 24199255. Archived from the original on 2017-03-14.
^ Hoyert DL, Xu J (October 2012). "Deaths: preliminary data for 2011". National Vital Statistics Reports. 61 (6): 1–51. PMID 24984457.
^ "Emphysema". Dictionary.com. Archived from the original on 24 November 2013. Retrieved 21 November 2013.
^ ab Ziment I (1991). "History of the treatment of chronic bronchitis". Respiration; International Review of Thoracic Diseases. 58 Suppl 1 (Suppl 1): 37–42. doi:10.1159/000195969. PMID 1925077.
^ abcd Petty TL (2006). "The history of COPD". International Journal of Chronic Obstructive Pulmonary Disease. 1 (1): 3–14. doi:10.2147/copd.2006.1.1.3. PMC 2706597. PMID 18046898.
^ ab Wright JL, Churg A (2008). "Pathologic Features of Chronic Obstructive Pulmonary Disease: Diagnostic Criteria and Differential Diagnosis" (PDF). In Fishman A, Elias J, Fishman J, Grippi M, Senior R, Pack A. Fishman's Pulmonary Diseases and Disorders (4th ed.). McGraw-Hill. pp. 693–705. ISBN 978-0-07-164109-8.
^ Waldbott GL (1965). A struggle with Titans. Carlton Press. p. 6.
^ Fishman AP (May 2005). "One hundred years of chronic obstructive pulmonary disease". American Journal of Respiratory and Critical Care Medicine. 171 (9): 941–8. doi:10.1164/rccm.200412-1685OE. PMID 15849329.
^ Yuh-Chin TH (2012). A clinical guide to occupational and environmental lung diseases. Humana Press. p. 266. ISBN 978-1-62703-149-3.
^ "Pink Puffer – definition of Pink Puffer in the Medical dictionary – by the Free Online Medical Dictionary, Thesaurus and Encyclopedia". Medical-dictionary.thefreedictionary.com. Retrieved 2013-07-23.
^ abc Weinberger SE (2013). Principles of pulmonary medicine (6th ed.). Elsevier/Saunders. p. 165. ISBN 978-1-62703-149-3.
^ Des Jardins T (2013). Clinical Manifestations & Assessment of Respiratory Disease (6th ed.). Elsevier Health Sciences. p. 176. ISBN 978-0-323-27749-5.
^ An outcomes strategy for people with chronic obstructive pulmonary disease (COPD) and asthma in England (PDF). Department of Health. 18 July 2011. p. 5. Archived (PDF) from the original on 5 December 2013. Retrieved 27 November 2013.
^ Bloom D (2011). The Global Economic Burden of Noncommunicable Diseases (PDF). World Economic Forum. p. 24. Archived (PDF) from the original on 2015-02-04.
^ Nici L (2011). Chronic Obstructive Pulmonary Disease: Co-Morbidities and Systemic Consequences. Springer. p. 78. ISBN 978-1-60761-673-3.
^ abc Chong, J; Leung, B; Poole, P (19 September 2017). "Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews. 9: CD002309. doi:10.1002/14651858.CD002309.pub5. PMID 28922692.
^ Inamdar AC, Inamdar AA (October 2013). "Mesenchymal stem cell therapy in lung disorders: pathogenesis of lung diseases and mechanism of action of mesenchymal stem cell". Experimental Lung Research. 39 (8): 315–27. doi:10.3109/01902148.2013.816803. PMID 23992090.
^ ab Oh DK, Kim YS, Oh YM (January 2017). "Lung Regeneration Therapy for Chronic Obstructive Pulmonary Disease". Tuberculosis and Respiratory Diseases. 80 (1): 1–10. doi:10.4046/trd.2017.80.1.1. PMC 5256352. PMID 28119741.
^ Conese M, Piro D, Carbone A, Castellani S, Di Gioia S (2014). "Hematopoietic and mesenchymal stem cells for the treatment of chronic respiratory diseases: role of plasticity and heterogeneity". TheScientificWorldJournal. 2014: 859817. doi:10.1155/2014/859817. PMC 3916026. PMID 24563632.
^ McQualter JL, Anthony D, Bozinovski S, Prêle CM, Laurent GJ (November 2014). "Harnessing the potential of lung stem cells for regenerative medicine". The International Journal of Biochemistry & Cell Biology. 56: 82–91. doi:10.1016/j.biocel.2014.10.012. PMID 25450456.
^ Tzouvelekis A, Ntolios P, Bouros D (2013). "Stem cell treatment for chronic lung diseases". Respiration; International Review of Thoracic Diseases. 85 (3): 179–92. doi:10.1159/000346525. PMID 23364286.
^ Tzouvelekis A, Laurent G, Bouros D (February 2013). "Stem cell therapy in chronic obstructive pulmonary disease. Seeking the Prometheus effect". Current Drug Targets. 14 (2): 246–52. doi:10.2174/1389450111314020009. PMID 23256721.
^ Gompelmann D, Eberhardt R, Herth FJ (August 2015). "Novel Endoscopic Approaches to Treating Chronic Obstructive Pulmonary Disease and Emphysema". Seminars in Respiratory and Critical Care Medicine. 36 (4): 609–15. doi:10.1055/s-0035-1555614. PMID 26238645.
^ Gøtzsche PC, Johansen HK (September 2016). "Intravenous alpha-1 antitrypsin augmentation therapy for treating patients with alpha-1 antitrypsin deficiency and lung disease". The Cochrane Database of Systematic Reviews. 9: CD007851. doi:10.1002/14651858.CD007851.pub3. PMID 27644166.
^ McLean S, Nurmatov U, Liu JL, Pagliari C, Car J, Sheikh A (July 2011). "Telehealthcare for chronic obstructive pulmonary disease". The Cochrane Database of Systematic Reviews (7): CD007718. doi:10.1002/14651858.CD007718.pub2. PMID 21735417.
^ Akers RM, Denbow DM (2008). Anatomy and Physiology of Domestic Animals. Wiley. p. 852. ISBN 978-1-118-70115-7.
^ Wright JL, Churg A (December 2002). "Animal models of cigarette smoke-induced COPD". Chest. 122 (6 Suppl): 301S–306S. doi:10.1378/chest.122.6_suppl.301S. PMID 12475805.
^ Churg A, Wright JL (2007). "Animal models of cigarette smoke-induced chronic obstructive lung disease". Contributions to Microbiology. Contributions to Microbiology. 14: 113–25. doi:10.1159/000107058. ISBN 3-8055-8332-X. PMID 17684336.
^ Marinkovic D, Aleksic-Kovacevic S, Plamenac P (2007). "Cellular basis of chronic obstructive pulmonary disease in horses". International Review of Cytology. International Review of Cytology. 257: 213–47. doi:10.1016/S0074-7696(07)57006-3. ISBN 978-0-12-373701-4. PMID 17280899.
^ Miller MS, Tilley LP, Smith FW (January 1989). "Cardiopulmonary disease in the geriatric dog and cat". The Veterinary Clinics of North America. Small Animal Practice. 19 (1): 87–102. PMID 2646821.
Further reading
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DM, López Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agusti A (April 2017). "Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary". Respirology. 22 (3): 575–601. doi:10.1111/resp.13012. PMID 28150362.
National Institute for Health and Clinical Excellence. Clinical guideline 101: Chronic Obstructive Pulmonary Disease. London, June 2010.
Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schünemann H, Wedzicha W, MacDonald R, Shekelle P (August 2011). "Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society". Annals of Internal Medicine. 155 (3): 179–91. doi:10.7326/0003-4819-155-3-201108020-00008. PMID 21810710.
External links
| Classification | D
|
|---|---|
| External resources |
|
| Wikimedia Commons has media related to Chronic obstructive pulmonary disease. |
Chronic obstructive pulmonary disease at Curlie (based on DMOZ)