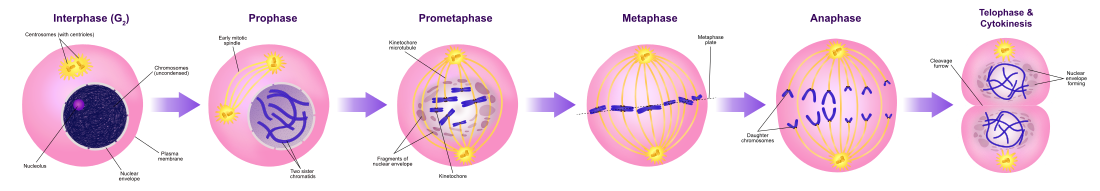
Mitosis

Mitosis in an animal cell (phases ordered counter-clockwise).

Mitosis divides the chromosomes in a cell nucleus.

Onion (Allium) cells in different phases of the cell cycle enlarged 800 diameters.
a. non-dividing cells
b. nuclei preparing for division (spireme-stage)
c. dividing cells showing mitotic figures
e. pair of daughter-cells shortly after division
In cell biology, mitosis (/maɪˈtoʊsɪs/) is a part of the cell cycle when replicated chromosomes are separated into two new nuclei. Cell division gives rise to genetically identical cells in which the number of chromosomes is maintained.[1] In general, mitosis (division of the nucleus) is preceded by the S stage of interphase (during which the DNA is replicated) and is often accompanied or followed by cytokinesis, which divides the cytoplasm, organelles and cell membrane into two new cells containing roughly equal shares of these cellular components.[2] Mitosis and cytokinesis together define the mitotic (M) phase of an animal cell cycle—the division of the mother cell into two daughter cells genetically identical to each other.
The process of mitosis is divided into stages corresponding to the completion of one set of activities and the start of the next. These stages are prophase, prometaphase, metaphase, anaphase, and telophase. During mitosis, the chromosomes, which have already duplicated, condense and attach to spindle fibers that pull one copy of each chromosome to opposite sides of the cell.[3] The result is two genetically identical daughter nuclei. The rest of the cell may then continue to divide by cytokinesis to produce two daughter cells.[4] Producing three or more daughter cells instead of the normal two is a mitotic error called tripolar mitosis or multipolar mitosis (direct cell triplication / multiplication).[5] Other errors during mitosis can induce apoptosis (programmed cell death) or cause mutations. Certain types of cancer can arise from such mutations.[6]
Mitosis occurs only in eukaryotic cells. Prokaryotic cells, which lack a nucleus, divide by a different process called binary fission. Mitosis varies between organisms.[7] For example, animal cells undergo an "open" mitosis, where the nuclear envelope breaks down before the chromosomes separate, whereas fungi undergo a "closed" mitosis, where chromosomes divide within an intact cell nucleus.[8] Most animal cells undergo a shape change, known as mitotic cell rounding, to adopt a near spherical morphology at the start of mitosis. Most human cells are produced by mitotic cell division. Important exceptions include the gametes – sperm and egg cells – which are produced by meiosis.
Contents
1 Discovery
2 Phases
2.1 Overview
2.2 Interphase
2.3 Mitosis
2.3.1 Preprophase (plant cells)
2.3.2 Prophase
2.3.3 Prometaphase
2.3.4 Metaphase
2.3.5 Anaphase
2.3.6 Telophase
2.4 Cytokinesis
3 Function
4 Variations
4.1 Forms of mitosis
4.2 Errors and other variations
5 Related cell processes
5.1 Cell rounding
5.2 Mitotic recombination
6 Evolution
7 Gallery
8 See also
9 References
10 Further reading
11 External links
Discovery
Numerous descriptions of cell division were made during 18th and 19th centuries, with various degrees of accuracy.[9] In 1835, the German botanist Hugo von Mohl, described cell division in the green alga Cladophora glomerata, stating that multiplication of cells occurs through cell division.[10][11][12] In 1838, Schleiden affirmed that the formation of new cells in their interior was a general law for cell multiplication in plants, a view later rejected in favour of Mohl model, due to contributions of Robert Remak and others.[13]
In animal cells, cell division with mitosis was discovered in frog, rabbit, and cat cornea cells in 1873 and described for the first time by the Polish histologist Wacław Mayzel in 1875.[14][15]
Bütschli, Schneider and Fol might have also claimed the discovery of the process presently known as "mitosis".[9] In 1873, the German zoologist Otto Bütschli published data from observations on nematodes. A few years later, he discovered and described mitosis based on those observations.[16][17][18]
The term "mitosis", coined by Walther Flemming in 1882,[19] is derived from the Greek word μίτος (mitos, "warp thread").[20][21] There are some alternative names for the process,[22] e.g., "karyokinesis" (nuclear division), a term introduced by Schleicher in 1878,[23][24] or "equational division", proposed by Weismann in 1887.[25] However, the term "mitosis" is also used in a broad sense by some authors to refer to karyokinesis and cytokinesis together.[26] Presently, "equational division" is more commonly used to refer to meiosis II, the part of meiosis most like mitosis.
Phases
Overview
 Play media
Play mediaTime-lapse video of mitosis in a Drosophila melanogaster embryo
The primary result of mitosis and cytokinesis is the transfer of a parent cell's genome into two daughter cells. The genome is composed of a number of chromosomes—complexes of tightly coiled DNA that contain genetic information vital for proper cell function. Because each resultant daughter cell should be genetically identical to the parent cell, the parent cell must make a copy of each chromosome before mitosis. This occurs during the S phase of interphase.[27]Chromosome duplication results in two identical sister chromatids bound together by cohesin proteins at the centromere.
When mitosis begins, the chromosomes condense and become visible. In some eukaryotes, for example animals, the nuclear envelope, which segregates the DNA from the cytoplasm, disintegrates into small vesicles. The nucleolus, which makes ribosomes in the cell, also disappears. Microtubules project from opposite ends of the cell, attach to the centromeres, and align the chromosomes centrally within the cell. The microtubules then contract to pull the sister chromatids of each chromosome apart.[28] Sister chromatids at this point are called daughter chromosomes. As the cell elongates, corresponding daughter chromosomes are pulled toward opposite ends of the cell and condense maximally in late anaphase. A new nuclear envelope forms around the separated daughter chromosomes, which decondense to form interphase nuclei.
During mitotic progression, typically after the anaphase onset, the cell may undergo cytokinesis. In animal cells, a cell membrane pinches inward between the two developing nuclei to produce two new cells. In plant cells, a cell plate forms between the two nuclei. Cytokinesis does not always occur; coenocytic (a type of multinucleate condition) cells undergo mitosis without cytokinesis.
Interphase
The mitotic phase is a relatively short period of the cell cycle. It alternates with the much longer interphase, where the cell prepares itself for the process of cell division. Interphase is divided into three phases: G1 (first gap), S (synthesis), and G2 (second gap). During all three parts of interphase, the cell grows by producing proteins and cytoplasmic organelles. However, chromosomes are replicated only during the S phase. Thus, a cell grows (G1), continues to grow as it duplicates its chromosomes (S), grows more and prepares for mitosis (G2), and finally divides (M) before restarting the cycle.[27] All these phases in the cell cycle are highly regulated by cyclins, cyclin-dependent kinases, and other cell cycle proteins. The phases follow one another in strict order and there are "checkpoints" that give the cell cues to proceed from one phase to another. Cells may also temporarily or permanently leave the cell cycle and enter G0 phase to stop dividing. This can occur when cells become overcrowded (density-dependent inhibition) or when they differentiate to carry out specific functions for the organism, as is the case for human heart muscle cells and neurons. Some G0 cells have the ability to re-enter the cell cycle.
Mitosis
Preprophase (plant cells)
In plant cells only, prophase is preceded by a pre-prophase stage. In highly vacuolated plant cells, the nucleus has to migrate into the center of the cell before mitosis can begin. This is achieved through the formation of a phragmosome, a transverse sheet of cytoplasm that bisects the cell along the future plane of cell division. In addition to phragmosome formation, preprophase is characterized by the formation of a ring of microtubules and actin filaments (called preprophase band) underneath the plasma membrane around the equatorial plane of the future mitotic spindle. This band marks the position where the cell will eventually divide. The cells of higher plants (such as the flowering plants) lack centrioles; instead, microtubules form a spindle on the surface of the nucleus and are then organized into a spindle by the chromosomes themselves, after the nuclear envelope breaks down.[29] The preprophase band disappears during nuclear envelope breakdown and spindle formation in prometaphase.[30]:58–67
Prophase
During prophase, which occurs after G2 interphase, the cell prepares to divide by tightly condensing its chromosomes and initiating mitotic spindle formation. During interphase, the genetic material in the nucleus consists of loosely packed chromatin. At the onset of prophase, chromatin fibers condense into discrete chromosomes that are typically visible at high magnification through a light microscope. In this stage, chromosomes are long, thin and thread-like. Each chromosome has two chromatids. The two chromatids are joined at the centromere.
Gene transcription ceases during prophase and does not resume until late anaphase to early G1 phase.[31][32][33] The nucleolus also disappears during early prophase.[34]

Condensing chromosomes. Interphase nucleus (left), condensing chromosomes (middle) and condensed chromosomes (right).
Close to the nucleus of animal cells are structures called centrosomes, consisting of a pair of centrioles surrounded by a loose collection of proteins. The centrosome is the coordinating center for the cell's microtubules. A cell inherits a single centrosome at cell division, which is duplicated by the cell before a new round of mitosis begins, giving a pair of centrosomes. The two centrosomes polymerize tubulin to help form a microtubule spindle apparatus. Motor proteins then push the centrosomes along these microtubules to opposite sides of the cell. Although centrosomes help organize microtubule assembly, they are not essential for the formation of the spindle apparatus, since they are absent from plants,[29] and are not absolutely required for animal cell mitosis.[35]
Prometaphase
At the beginning of prometaphase in animal cells, phosphorylation of nuclear lamins causes the nuclear envelope to disintegrate into small membrane vesicles. As this happens, microtubules invade the nuclear space. This is called open mitosis, and it occurs in some multicellular organisms. Fungi and some protists, such as algae or trichomonads, undergo a variation called closed mitosis where the spindle forms inside the nucleus, or the microtubules penetrate the intact nuclear envelope.[36][37]
In late prometaphase, kinetochore microtubules begin to search for and attach to chromosomal kinetochores.[38] A kinetochore is a proteinaceous microtubule-binding structure that forms on the chromosomal centromere during late prophase.[38][39] A number of polar microtubules find and interact with corresponding polar microtubules from the opposite centrosome to form the mitotic spindle.[40] Although the kinetochore structure and function are not fully understood, it is known that it contains some form of molecular motor.[41] When a microtubule connects with the kinetochore, the motor activates, using energy from ATP to "crawl" up the tube toward the originating centrosome. This motor activity, coupled with polymerisation and depolymerisation of microtubules, provides the pulling force necessary to later separate the chromosome's two chromatids.[41]
Metaphase
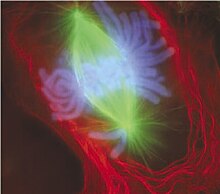
A cell in late metaphase. All chromosomes (blue) but one have arrived at the metaphase plate.
After the microtubules have located and attached to the kinetochores in prometaphase, the two centrosomes begin pulling the chromosomes towards opposite ends of the cell. The resulting tension causes the chromosomes to align along the metaphase plate or equatorial plane, an imaginary line that is centrally located between the two centrosomes (at approximately the midline of the cell).[40] To ensure equitable distribution of chromosomes at the end of mitosis, the metaphase checkpoint guarantees that kinetochores are properly attached to the mitotic spindle and that the chromosomes are aligned along the metaphase plate.[42] If the cell successfully passes through the metaphase checkpoint, it proceeds to anaphase.
Anaphase
During anaphase A, the cohesins that bind sister chromatids together are cleaved, forming two identical daughter chromosomes.[43] Shortening of the kinetochore microtubules pulls the newly formed daughter chromosomes to opposite ends of the cell. During anaphase B, polar microtubules push against each other, causing the cell to elongate.[44] In late anaphase, chromosomes also reach their overall maximal condensation level, to help chromosome segregation and the re-formation of the nucleus.[45] In most animal cells, anaphase A precedes anaphase B, but some vertebrate egg cells demonstrate the opposite order of events.[43]
Telophase
Telophase (from the Greek word τελος meaning "end") is a reversal of prophase and prometaphase events. At telophase, the polar microtubules continue to lengthen, elongating the cell even more. If the nuclear envelope has broken down, a new nuclear envelope forms using the membrane vesicles of the parent cell's old nuclear envelope. The new envelope forms around each set of separated daughter chromosomes (though the membrane does not enclose the centrosomes) and the nucleolus reappears. Both sets of chromosomes, now surrounded by new nuclear membrane, begin to "relax" or decondense. Mitosis is complete. Each daughter nucleus has an identical set of chromosomes. Cell division may or may not occur at this time depending on the organism.
Cytokinesis
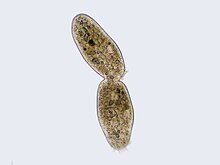
Cilliate undergoing cytokinesis, with the cleavage furrow being clearly visible
Cytokinesis is not a phase of mitosis but rather a separate process, necessary for completing cell division. In animal cells, a cleavage furrow (pinch) containing a contractile ring develops where the metaphase plate used to be, pinching off the separated nuclei.[46] In both animal and plant cells, cell division is also driven by vesicles derived from the Golgi apparatus, which move along microtubules to the middle of the cell.[47] In plants, this structure coalesces into a cell plate at the center of the phragmoplast and develops into a cell wall, separating the two nuclei. The phragmoplast is a microtubule structure typical for higher plants, whereas some green algae use a phycoplast microtubule array during cytokinesis.[30]:64–7, 328–9 Each daughter cell has a complete copy of the genome of its parent cell. The end of cytokinesis marks the end of the M-phase.
There are many cells where mitosis and cytokinesis occur separately, forming single cells with multiple nuclei. The most notable occurrence of this is among the fungi, slime molds, and coenocytic algae, but the phenomenon is found in various other organisms. Even in animals, cytokinesis and mitosis may occur independently, for instance during certain stages of fruit fly embryonic development.[48]
Function
Mitosis "function" or significance relies on the maintenance of the chromosomal set; each cell formed receives chromosomes that are alike in composition and equal in number to the chromosomes of the parent cell.
Mitosis occurs in the following circumstances:
- Development and growth
- The number of cells within an organism increases by mitosis. This is the basis of the development of a multicellular body from a single cell, i.e., zygote and also the basis of the growth of a multicellular body.
- Cell replacement
- In some parts of body, e.g. skin and digestive tract, cells are constantly sloughed off and replaced by new ones. New cells are formed by mitosis and so are exact copies of the cells being replaced. In like manner, red blood cells have short lifespan (only about 4 months) and new RBCs are formed by mitosis.
- Regeneration
- Some organisms can regenerate body parts. The production of new cells in such instances is achieved by mitosis. For example, starfish regenerate lost arms through mitosis.
- Asexual reproduction
- Some organisms produce genetically similar offspring through asexual reproduction. For example, the hydra reproduces asexually by budding. The cells at the surface of hydra undergo mitosis and form a mass called a bud. Mitosis continues in the cells of the bud and this grows into a new individual. The same division happens during asexual reproduction or vegetative propagation in plants.
Variations
Forms of mitosis
The mitosis process in the cells of eukaryotic organisms follow a similar pattern, but with variations in three main details. "Closed" and "open" mitosis can be distinguished on the basis of nuclear envelope remaining intact or breaking down. An intermediate form with partial degradation of the nuclear envelope is called a "semiopen" mitosis. With respesct to the symmetry of the spindle apparatus during metaphase, an approximately axially symmetric (centered) shape is called as "orthomitosis", distinguished from the eccentric spindles of "pleuromitosis", in which mitotic apparatus has a bilateral symmetry. Finally, a third criterion is the location of the central spindle in case of closed pleuromitosis: "extranuclear" (spindle located in the cytoplasm) or "intranuclear" (in the nucleus).[7]
closed
intranuclear
pleuromitosis

closed
extranuclear
pleuromitosis
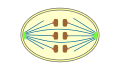
closed
orthomitosis

semiopen
pleuromitosis
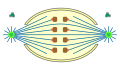
semiopen
orthomitosis
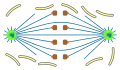
open
orthomitosis
Nuclear division takes place only in cells of organisms of the eukaryotic domain, as bacteria and archaea have no nucleus. Within each of the eukaryotic supergroups, mitosis of the open form can be found, as well as closed mitosis, except for Excavata, which show exclusively closed mitosis.[49] Following, the occurrence of the forms of mitosis in eukaryotes:[7][50]
Closed intranuclear pleuromitosis is typical of Foraminifera, some Prasinomonadida, some Kinetoplastida, the Oxymonadida, the Haplosporidia, many fungi (chytrids, oomycetes, zygomycetes, ascomycetes), and some Radiolaria (Spumellaria and Acantharia); it seems to be the most primitive type.
Closed extranuclear pleuromitosis occurs in Trichomonadida and Dinoflagellata.
Closed orthomitosis is found among diatoms, ciliates, some Microsporidia, unicellular yeasts and some multicellular fungi.
Semiopen pleuromitosis is typical of most Apicomplexa.
Semiopen orthomitosis occurs with different variants in some amoebae (Lobosa) and some green flagellates (e.g., Raphidophyta or Volvox).
Open orthomitosis is typical in mammals and other Metazoa, and in land plants; but it also occurs in some protists.
Errors and other variations
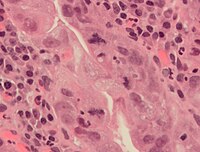
An abnormal (tripolar) mitosis (12 o'clock position) in a precancerous lesion of the stomach (H&E stain)
Errors can occur during mitosis, especially during early embryonic development in humans.[51] Mitotic errors can create aneuploid cells that have too few or too many of one or more chromosomes, a condition associated with cancer.[52][53] Early human embryos, cancer cells, infected or intoxicated cells can also suffer from pathological division into three or more daughter cells (tripolar or multipolar mitosis), resulting in severe errors in their chromosomal complements.[5]
In nondisjunction, sister chromatids fail to separate during anaphase.[54] One daughter cell receives both sister chromatids from the nondisjoining chromosome and the other cell receives none. As a result, the former cell gets three copies of the chromosome, a condition known as trisomy, and the latter will have only one copy, a condition known as monosomy. On occasion, when cells experience nondisjunction, they fail to complete cytokinesis and retain both nuclei in one cell, resulting in binucleated cells.[55]
Anaphase lag occurs when the movement of one chromatid is impeded during anaphase.[54] This may be caused by a failure of the mitotic spindle to properly attach to the chromosome. The lagging chromatid is excluded from both nuclei and is lost. Therefore, one of the daughter cells will be monosomic for that chromosome.
Endoreduplication (or endoreplication) occurs when chromosomes duplicate but the cell does not subsequently divide. This results in polyploid cells or, if the chromosomes duplicates repeatedly, polytene chromosomes.[54][56] Endoreduplication is found in many species and appears to be a normal part of development.[56] Endomitosis is a variant of endoreduplication in which cells replicate their chromosomes during S phase and enter, but prematurely terminate, mitosis. Instead of being divided into two new daughter nuclei, the replicated chromosomes are retained within the original nucleus.[48][57] The cells then re-enter G1 and S phase and replicate their chromosomes again.[57] This may occur multiple times, increasing the chromosome number with each round of replication and endomitosis. Platelet-producing megakaryocytes go through endomitosis during cell differentiation.[58][59]
Amitosis in ciliates and in animal placental tissues results in a random distribution of parental alleles.
Karyokinesis without cytokinesis originates multinucleated cells called coenocytes.
Related cell processes
Cell rounding

Cell shape changes through mitosis for a typical animal cell cultured on a flat surface. The cell undergoes mitotic cell rounding during spindle assembly and then divides via cytokinesis. The actomyosin cortex is depicted in red, DNA/chromosomes purple, microtubules green, and membrane and retraction fibers in black. Rounding also occurs in live tissue, as described in the text.
In animal tissue, most cells round up to a near-spherical shape during mitosis.[60][61][62] In epithelia and epidermis, an efficient rounding process is correlated with proper mitotic spindle alignment and subsequent correct positioning of daughter cells.[61][62][63][64] Moreover, researchers have found that if rounding is heavily suppressed it may result in spindle defects, primarily pole splitting and failure to efficiently capture chromosomes.[65] Therefore, mitotic cell rounding is thought to play a protective role in ensuring accurate mitosis.[64][66]
Rounding forces are driven by reorganization of F-actin and myosin (actomyosin) into a contractile homogeneous cell cortex that 1) rigidifies the cell periphery[66][67][68] and 2) facilitates generation of intracellular hydrostatic pressure (up to 10 fold higher than interphase).[69][70][71] The generation of intracellular pressure is particularly critical under confinement, such as would be important in a tissue scenario, where outward forces must be produced to round up against surrounding cells and/or the extracellular matrix. Generation of pressure is dependent on formin-mediated F-actin nucleation[71] and Rho kinase (ROCK)-mediated myosin II contraction,[67][69][71] both of which are governed upstream by signaling pathways RhoA and ECT2[67][68] through the activity of Cdk1.[71] Due to its importance in mitosis, the molecular components and dynamics of the mitotic actomyosin cortex is an area of active research.
Mitotic recombination
Mitotic cells irradiated with X-rays in the G1 phase of the cell cycle repair recombinogenic DNA damages primarily by recombination between homologous chromosomes.[72] Mitotic cells irradiated in the G2 phase repair such damages preferentially by sister-chromatid recombination.[72]Mutations in genes encoding enzymes employed in recombination cause cells to have increased sensitivity to being killed by a variety of DNA damaging agents.[73] These findings suggest that mitotic recombination is an adaptation for repairing DNA damages including those that are potentially lethal.
Evolution
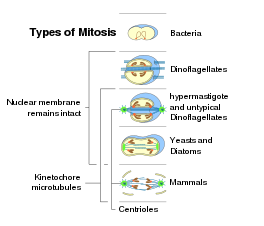
Some types of cell division in prokaryotes and eukaryotes
There are prokaryotic homologs of all the key molecules of eukaryotic mitosis (e.g., actins, tubulins). Being a universal eukaryotic property, mitosis probably arose at the base of the eukaryotic tree. As mitosis is less complex than meiosis, meiosis presumably arose after mitosis.[74]
While in bacterial cell division, after duplication of DNA, two circular chromosomes are attached to a special region of the cell membrane, eukaryotic mitosis is usually characterized by the presence of many linear chromosomes, whose kinetochores attaches to the microtubules of the spindle. In relation to the forms of mitosis, closed intranuclear pleuromitosis seems to be the most primitive type, as it is the more similar to bacterial division.[7]
Gallery
Mitotic cells can be visualized microscopically by staining them with fluorescent antibodies and dyes.
.mw-parser-output .mod-gallery{display:table}.mw-parser-output .mod-gallery-default{background:transparent;margin-top:0.5em}.mw-parser-output .mod-gallery-center{margin-left:auto;margin-right:auto}.mw-parser-output .mod-gallery-left{float:left}.mw-parser-output .mod-gallery-right{float:right}.mw-parser-output .mod-gallery-none{float:none}.mw-parser-output .mod-gallery-collapsible{width:100%}.mw-parser-output .mod-gallery .title{display:table-row}.mw-parser-output .mod-gallery .title>div{display:table-cell;text-align:center;font-weight:bold}.mw-parser-output .mod-gallery .main{display:table-row}.mw-parser-output .mod-gallery .main>div{display:table-cell}.mw-parser-output .mod-gallery .caption{display:table-row;vertical-align:top}.mw-parser-output .mod-gallery .caption>div{display:table-cell;display:block;font-size:94%;padding:0}.mw-parser-output .mod-gallery .footer{display:table-row}.mw-parser-output .mod-gallery .footer>div{display:table-cell;text-align:right;font-size:80%;line-height:1em}.mw-parser-output .mod-gallery .gallerybox .thumb img{background:none}.mw-parser-output .mod-gallery .bordered-images img{border:solid #eee 1px}.mw-parser-output .mod-gallery .whitebg img{background:#fff!important}.mw-parser-output .mod-gallery .gallerybox div{background:#fff!important}

Early prophase: Polar microtubules, shown as green strands, have established a matrix around the currently intact nucleus, with the condensing chromosomes in blue. The red nodules are the centromeres.
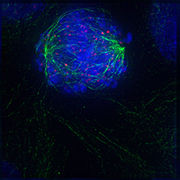
Early prometaphase: The nuclear membrane has just disassembled, allowing the microtubules to quickly interact with the kinetochores, which assemble on the centromeres of the condensing chromosomes.
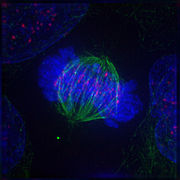
Metaphase: The centrosomes have moved to the poles of the cell and have established the mitotic spindle. The chromosomes have congressed at the metaphase plate.

Anaphase: Kinetochore microtubules pull the two sets of chromosomes apart, and lengthening polar microtubules push the halves of the dividing cell further apart, while chromosomes are condensed maximally.
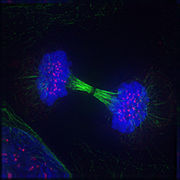
Telophase: Reversal of prophase and prometaphase events and thus completing the cell cycle.
See also
- Aneuploidy
- Binary fission
- Chromosome abnormality
- Cytoskeleton
- Meiosis
- Mitogen
- Mitosis Promoting Factor
- Mitotic bookmarking
- Motor protein
References
^ "Cell division and growth". britannica.com. ENCYCLOPÆDIA BRITANNICA..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Carter, J. Stein (2014-01-14). "Mitosis". biology.clc.uc.edu. Archived from the original on 2012-10-27.
^ "Cell Division: Stages of Mitosis | Learn Science at Scitable". www.nature.com. Archived from the original on 2015-11-14. Retrieved 2015-11-16.
^ Maton A, Hopkins JJ, LaHart S, Quon Warner D, Wright M, Jill D (1997). Cells: Building Blocks of Life. New Jersey: Prentice Hall. pp. 70–4. ISBN 978-0-13-423476-2.
^ ab Kalatova B, Jesenska R, Hlinka D, Dudas M (January 2015). "Tripolar mitosis in human cells and embryos: occurrence, pathophysiology and medical implications". Acta Histochemica. 117 (1): 111–25. doi:10.1016/j.acthis.2014.11.009. PMID 25554607.
^ Kops GJ, Weaver BA, Cleveland DW (October 2005). "On the road to cancer: aneuploidy and the mitotic checkpoint". Nature Reviews. Cancer. 5 (10): 773–85. doi:10.1038/nrc1714. PMID 16195750.
^ abcd Raikov, IB (1994). "The diversity of forms of mitosis in protozoa: A comparative review". European Journal of Protistology. 30 (3): 253–69. doi:10.1016/S0932-4739(11)80072-6.
^ De Souza CP, Osmani SA (September 2007). "Mitosis, not just open or closed". Eukaryotic Cell. 6 (9): 1521–7. doi:10.1128/EC.00178-07. PMC 2043359. PMID 17660363.
^ ab Ross, Anna E. "Human Anatomy & Physiology I: A Chronology of the Description of Mitosis". Christian Brothers University. Retrieved 02 May 2018. link.
^ Mohl, Hugo von (1835). Ueber die Vermehrung der Pflanzenzellen durch Theilung. Inaugural-Dissertation, Tübingen. link.
^ Karl Mägdefrau (1994), "Mohl, Hugo von", Neue Deutsche Biographie (NDB) (in German), 17, Berlin: Duncker & Humblot, pp. 690–691; (full text online)
^ "Notes and memoranda: The late professor von Mohl". Quarterly Journal of Microscopical Science, v. XV, New Series, p. 178-181, 1875. link.
^ Weyers, Wolfgang (2002). 150 Years of cell division. Dermatopathology: Practical & Conceptual, Vol. 8, No. 2. link.
^ Janusz Komender (2008). "Kilka słów o doktorze Wacławie Mayzlu i jego odkryciu" [On Waclaw Mayzel and his observation of mitotic division] (PDF). Postępy Biologii Komórki (in Polish). 35 (3): 405–407. Archived (PDF) from the original on 2012-10-27.
^ Iłowiecki M (1981). Dzieje nauki polskiej. Warszawa: Wydawnictwo Interpress. p. 187. ISBN 978-83-223-1876-8.
^ Bütschli, O. (1873). Beiträge zur Kenntnis der freilebenden Nematoden. Nova Acta der Kaiserlich Leopoldinisch-Carolinischen Deutschen Akademie der Naturforscher 36, 1-144. link.
^ Bütschli, O. (1876). Studien über die ersten Entwicklungsvorgänge der Eizelle, die Zelleilung und die Conjugation der Infusorien. Abh.d. Senckenb. Naturf. Ges. Frankfurt a. M. 10, 213-452. link.
^ Fokin, S. I. (2013). "Otto Bütschli (1848–1920) Where we will genuflect?" (PDF). Protistology. 8 (1): 22–35.
^ Sharp LW (1921). Introduction To Cytology. New York: McGraw Hill Book Company Inc. p. 143.
^ "mitosis". Online Etymology Dictionary.
^ μίτος. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project
^ Battaglia, Emilio (2009). Caryoneme alternative to chromosome and a new caryological nomenclature. Caryologia 62 (4): 1–80. link.
^ Schleicher, W. (1878). Die Knorpelzelltheilung. Arch. Mirkroskop. Anat. 16, 248-300. link.
^ Toepfer, G. "Karyokinesis". BioConcepts. Retrieved 02 May 2018. link.
^ Battaglia E. (1987). Embryological questions: 12. Have the Polygonum and Allium types been rightly established? Ann Bot (Rome) 45: 81–117. [p. 85: "Already in 1887, Weismann gave the names Aequationstheilung to the usual cell division, and Reduktionstheilungen to the two divisions involved in the halving process of the number of Kernsegmente.]"
^ Mauseth, J.D. (1991). Botany: an Introduction to Plant Biology. Saunders College Publishing, Philadelphia. [p. 102: "Cell division is cytokinesis, and nuclear division is karyokinesis. The words "mitosis" and “meiosis" technically refer only to karyokinesis but are frequently used to describe cytokinesis as well."] link.
^ ab Blow JJ, Tanaka TU (November 2005). "The chromosome cycle: coordinating replication and segregation. Second in the cycles review series". EMBO Reports. 6 (11): 1028–34. doi:10.1038/sj.embor.7400557. PMC 1371039. PMID 16264427.
^ Zhou J, Yao J, Joshi HC (September 2002). "Attachment and tension in the spindle assembly checkpoint". Journal of Cell Science. 115 (Pt 18): 3547–55. doi:10.1242/jcs.00029. PMID 12186941.
^ ab Lloyd C, Chan J (February 2006). "Not so divided: the common basis of plant and animal cell division". Nature Reviews Molecular Cell Biology. 7 (2): 147–52. doi:10.1038/nrm1831. PMID 16493420.
^ ab Raven PH, Evert RF, Eichhorn SE (2005). Biology of Plants (7th ed.). New York: W. H. Freeman and Co. ISBN 978-0716710073.
^ Prasanth KV, Sacco-Bubulya PA, Prasanth SG, Spector DL (March 2003). "Sequential entry of components of the gene expression machinery into daughter nuclei". Molecular Biology of the Cell. 14 (3): 1043–57. doi:10.1091/mbc.E02-10-0669. PMC 151578. PMID 12631722.
^ Kadauke S, Blobel GA (April 2013). "Mitotic bookmarking by transcription factors". Epigenetics & Chromatin. 6 (1): 6. doi:10.1186/1756-8935-6-6. PMC 3621617. PMID 23547918.
^ Prescott DM, Bender MA (March 1962). "Synthesis of RNA and protein during mitosis in mammalian tissue culture cells". Experimental Cell Research. 26 (2): 260–8. doi:10.1016/0014-4827(62)90176-3. PMID 14488623.
^ Olson MO (2011). The Nucleolus. Volume 15 of Protein Reviews. Berlin: Springer Science & Business Media. p. 15. ISBN 9781461405146.
^ Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW (June 2006). "Flies without centrioles". Cell. 125 (7): 1375–86. doi:10.1016/j.cell.2006.05.025. PMID 16814722.
^ Heywood P (June 1978). "Ultrastructure of mitosis in the chloromonadophycean alga Vacuolaria virescens". Journal of Cell Science. 31: 37–51. PMID 670329.
^ Ribeiro KC, Pereira-Neves A, Benchimol M (June 2002). "The mitotic spindle and associated membranes in the closed mitosis of trichomonads". Biology of the Cell. 94 (3): 157–72. doi:10.1016/S0248-4900(02)01191-7. PMID 12206655.
^ ab Chan GK, Liu ST, Yen TJ (November 2005). "Kinetochore structure and function". Trends in Cell Biology. 15 (11): 589–98. doi:10.1016/j.tcb.2005.09.010. PMID 16214339.
^ Cheeseman IM, Desai A (January 2008). "Molecular architecture of the kinetochore-microtubule interface". Nature Reviews Molecular Cell Biology. 9 (1): 33–46. doi:10.1038/nrm2310. PMID 18097444.
^ ab Winey M, Mamay CL, O'Toole ET, Mastronarde DN, Giddings TH, McDonald KL, McIntosh JR (June 1995). "Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle". The Journal of Cell Biology. 129 (6): 1601–15. doi:10.1083/jcb.129.6.1601. PMC 2291174. PMID 7790357.
^ ab Maiato H, DeLuca J, Salmon ED, Earnshaw WC (November 2004). "The dynamic kinetochore-microtubule interface" (PDF). Journal of Cell Science. 117 (Pt 23): 5461–77. doi:10.1242/jcs.01536. PMID 15509863.
^ Chan GK, Yen TJ (2003). "The mitotic checkpoint: a signaling pathway that allows a single unattached kinetochore to inhibit mitotic exit". Progress in Cell Cycle Research. 5: 431–9. PMID 14593737.
^ ab FitzHarris G (March 2012). "Anaphase B precedes anaphase A in the mouse egg". Current Biology. 22 (5): 437–44. doi:10.1016/j.cub.2012.01.041. PMID 22342753.

^ Miller KR (2000). "Anaphase". Biology (5 ed.). Pearson Prentice Hall. pp. 169–70. ISBN 978-0-13-436265-6.
^ "Chromosome condensation through mitosis". Science Daily. Retrieved 12 June 2007.
^ Glotzer M (March 2005). "The molecular requirements for cytokinesis". Science. 307 (5716): 1735–9. doi:10.1126/science.1096896. PMID 15774750.
^ Albertson R, Riggs B, Sullivan W (February 2005). "Membrane traffic: a driving force in cytokinesis". Trends in Cell Biology. 15 (2): 92–101. doi:10.1016/j.tcb.2004.12.008. PMID 15695096.
^ ab Lilly MA, Duronio RJ (April 2005). "New insights into cell cycle control from the Drosophila endocycle". Oncogene. 24 (17): 2765–75. doi:10.1038/sj.onc.1208610. PMID 15838513.
^ Boettcher, B.; Barral, Y. (2013). "The cell biology of open and closed mitosis". Nucleus. 4 (3): 160–5. doi:10.4161/nucl.24676. PMC 3720745. PMID 23644379.
^ R. Desalle, B. Schierwater: Key Transitions in Animal Evolution. CRC Press, 2010, p. 12, link.
^ Mantikou E, Wong KM, Repping S, Mastenbroek S (December 2012). "Molecular origin of mitotic aneuploidies in preimplantation embryos". Biochimica et Biophysica Acta. 1822 (12): 1921–30. doi:10.1016/j.bbadis.2012.06.013. PMID 22771499.
^ Draviam VM, Xie S, Sorger PK (April 2004). "Chromosome segregation and genomic stability". Current Opinion in Genetics & Development. 14 (2): 120–5. doi:10.1016/j.gde.2004.02.007. PMID 15196457.
^ Santaguida, Stefano; Amon, Angelika (2015-08-01). "Short- and long-term effects of chromosome mis-segregation and aneuploidy". Nature Reviews. Molecular Cell Biology. 16 (8): 473–485. doi:10.1038/nrm4025. hdl:1721.1/117201. ISSN 1471-0080. PMID 26204159.
^ abc Iourov I, Vorsanova S, Yurov Y (2006). "Chromosomal Variations in Mammalian Neuronal Cells: Known Facts and Attractive Hypotheses". In Jeon KJ. International Review Of Cytology: A Survey of Cell Biology. 249. Waltham, MA: Academic Press. p. 146. ISBN 9780080463506.
^ Shi Q, King RW (October 2005). "Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines". Nature. 437 (7061): 1038–42. doi:10.1038/nature03958. PMID 16222248.
^ ab Edgar BA, Orr-Weaver TL (May 2001). "Endoreplication cell cycles: more for less". Cell. 105 (3): 297–306. doi:10.1016/S0092-8674(01)00334-8. PMID 11348589.
^ ab Lee HO, Davidson JM, Duronio RJ (November 2009). "Endoreplication: polyploidy with purpose". Genes & Development. 23 (21): 2461–77. doi:10.1101/gad.1829209. PMC 2779750. PMID 19884253.
^ Italiano JE, Shivdasani RA (June 2003). "Megakaryocytes and beyond: the birth of platelets". Journal of Thrombosis and Haemostasis. 1 (6): 1174–82. doi:10.1046/j.1538-7836.2003.00290.x. PMID 12871316.
^ Vitrat N, Cohen-Solal K, Pique C, Le Couedic JP, Norol F, Larsen AK, Katz A, Vainchenker W, Debili N (May 1998). "Endomitosis of human megakaryocytes are due to abortive mitosis". Blood. 91 (10): 3711–23. PMID 9573008.
^ Sauer, F. C. (1935). "Mitosis in the neural tube". Journal of Comparative Neurology. 62 (2): 377–405. doi:10.1002/cne.900620207.
^ ab Meyer EJ, Ikmi A, Gibson MC (March 2011). "Interkinetic nuclear migration is a broadly conserved feature of cell division in pseudostratified epithelia". Current Biology. 21 (6): 485–91. doi:10.1016/j.cub.2011.02.002. PMID 21376598.
^ ab Luxenburg C, Pasolli HA, Williams SE, Fuchs E (March 2011). "Developmental roles for Srf, cortical cytoskeleton and cell shape in epidermal spindle orientation". Nature Cell Biology. 13 (3): 203–14. doi:10.1038/Ncb2163. PMC 3278337. PMID 21336301.
^ Nakajima Y, Meyer EJ, Kroesen A, McKinney SA, Gibson MC (August 2013). "Epithelial junctions maintain tissue architecture by directing planar spindle orientation". Nature. 500 (7462): 359–62. doi:10.1038/nature12335. PMID 23873041.
^ ab Cadart C, Zlotek-Zlotkiewicz E, Le Berre M, Piel M, Matthews HK (April 2014). "Exploring the function of cell shape and size during mitosis". Developmental Cell. 29 (2): 159–69. doi:10.1016/j.devcel.2014.04.009. PMID 24780736.
^ Lancaster OM, Le Berre M, Dimitracopoulos A, Bonazzi D, Zlotek-Zlotkiewicz E, Picone R, Duke T, Piel M, Baum B (May 2013). "Mitotic rounding alters cell geometry to ensure efficient bipolar spindle formation". Developmental Cell. 25 (3): 270–83. doi:10.1016/j.devcel.2013.03.014. PMID 23623611.
^ ab Lancaster OM, Baum B (October 2014). "Shaping up to divide: coordinating actin and microtubule cytoskeletal remodelling during mitosis". Seminars in Cell & Developmental Biology. 34: 109–15. doi:10.1016/j.semcdb.2014.02.015. PMID 24607328.
^ abc Maddox AS, Burridge K (January 2003). "RhoA is required for cortical retraction and rigidity during mitotic cell rounding". The Journal of Cell Biology. 160 (2): 255–65. doi:10.1083/jcb.200207130. PMC 2172639. PMID 12538643.
^ ab Matthews HK, Delabre U, Rohn JL, Guck J, Kunda P, Baum B (August 2012). "Changes in Ect2 localization couple actomyosin-dependent cell shape changes to mitotic progression". Developmental Cell. 23 (2): 371–83. doi:10.1016/j.devcel.2012.06.003. PMC 3763371. PMID 22898780.
^ ab Stewart MP, Helenius J, Toyoda Y, Ramanathan SP, Muller DJ, Hyman AA (January 2011). "Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding". Nature. 469 (7329): 226–30. doi:10.1038/nature09642. PMID 21196934.
^ Fischer-Friedrich E, Hyman AA, Jülicher F, Müller DJ, Helenius J (August 2014). "Quantification of surface tension and internal pressure generated by single mitotic cells". Scientific Reports. 4 (6213): 6213. doi:10.1038/srep06213. PMC 4148660. PMID 25169063.
^ abcd Ramanathan SP, Helenius J, Stewart MP, Cattin CJ, Hyman AA, Muller DJ (February 2015). "Cdk1-dependent mitotic enrichment of cortical myosin II promotes cell rounding against confinement". Nature Cell Biology. 17 (2): 148–59. doi:10.1038/ncb3098. PMID 25621953.
^ ab Kadyk LC, Hartwell LH (1992). "Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae". Genetics. 132 (2): 387–402. PMC 1205144. PMID 1427035.
^ Harris Bernstein, Carol Bernstein and Richard E. Michod (2011). Meiosis as an Evolutionary Adaptation for DNA Repair. Chapter 19 in DNA Repair. Inna Kruman editor. InTech Open Publisher. DOI: 10.5772/25117 Bernstein, Harris; Bernstein, Carol; e, Richard (2011). "Meiosis as an Evolutionary Adaptation for DNA Repair". DNA Repair. doi:10.5772/25117. ISBN 978-953-307-697-3. Archived from the original on 2013-06-16. Retrieved 2013-07-29.
^ Wilkins AS, Holliday R (January 2009). "The evolution of meiosis from mitosis". Genetics. 181 (1): 3–12. doi:10.1534/genetics.108.099762. PMC 2621177. PMID 19139151.
Further reading
.mw-parser-output .refbegin{font-size:90%;margin-bottom:0.5em}.mw-parser-output .refbegin-hanging-indents>ul{list-style-type:none;margin-left:0}.mw-parser-output .refbegin-hanging-indents>ul>li,.mw-parser-output .refbegin-hanging-indents>dl>dd{margin-left:0;padding-left:3.2em;text-indent:-3.2em;list-style:none}.mw-parser-output .refbegin-100{font-size:100%}
Morgan DL (2007). The cell cycle: principles of control. London: Published by New Science Press in association with Oxford University Press. ISBN 978-0-9539181-2-6.
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "Mitosis". Molecular Biology of the Cell (4th ed.). Garland Science. Retrieved 2006-01-22.
Campbell N, Reece J (December 2001). "The Cell Cycle". Biology (6th ed.). San Francisco: Benjamin Cummings/Addison-Wesley. pp. 217–224. ISBN 978-0-8053-6624-2.
Cooper G (2000). "The Events of M Phase". The Cell: A Molecular Approach (2nd ed.). Sinaeur Associates, Inc. Retrieved 2006-01-22.
Freeman S (2002). "Cell Division". Biological Science. Upper Saddle River, NJ: Prentice Hall. pp. 155–174. ISBN 978-0-13-081923-9.
Lodish H, Berk A, Zipursky L, Matsudaira P, Baltimore D, Darnell J (2000). "Overview of the Cell Cycle and Its Control". Molecular Cell Biology (4th ed.). W. H. Freeman. Retrieved 2006-01-22.
External links
| Wikimedia Commons has media related to Mitosis. |
| Wikiversity has learning resources about Overview of Cell Biology/Mitosis |
- A Flash animation comparing Mitosis and Meiosis
- Khan Academy, lecture
- Studying Mitosis in Cultured Mammalian Cells
- General K-12 classroom resources for Mitosis
- The Cell-Cycle Ontology
WormWeb.org: Interactive Visualization of the C. elegans Cell Lineage – Visualize the entire cell lineage tree and all of the cell divisions of the nematode C. elegans