Vitamin K
Vitamin K
Jump to navigation
Jump to search
| Vitamin K | |
|---|---|
| Drug class | |
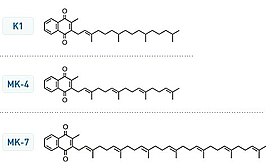 Vitamin K structures. MK-4 and MK-7 are both subtypes of K2. | |
| Class identifiers | |
| Use | Vitamin K deficiency, Warfarin overdose |
| ATC code | B02BA |
| Biological target | Gamma-glutamyl carboxylase |
| Clinical data | |
| Drugs.com | Medical Encyclopedia |
| External links | |
| MeSH | D014812 |
| In Wikidata | |
Vitamin K is a group of structurally similar, fat-soluble vitamins that the human body requires for complete synthesis of certain proteins that are prerequisites for blood coagulation (K from Koagulation, German for "coagulation") and which the body also needs for controlling binding of calcium in bones and other tissues.[1] The vitamin K-related modification of the proteins allows them to bind calcium ions, which they cannot do otherwise. Without vitamin K, blood coagulation is seriously impaired, and uncontrolled bleeding occurs. Preliminary clinical research indicates that deficiency of vitamin K may weaken bones, potentially leading to osteoporosis, and may promote calcification of arteries and other soft tissues.[1]
Chemically, the vitamin K family comprises 2-methyl-1,4-naphthoquinone (3-) derivatives. Vitamin K includes two natural vitamers: vitamin K1 and vitamin K2.[2] Vitamin K2, in turn, consists of a number of related chemical subtypes, with differing lengths of carbon side chains made of isoprenoid groups of atoms.
Vitamin K1, also known as phylloquinone, is made by plants, and is found in highest amounts in green leafy vegetables because it is directly involved in photosynthesis. It may be thought of as the plant form of vitamin K. It is active as a vitamin in animals and performs the classic functions of vitamin K, including its activity in the production of blood-clotting proteins. Animals may also convert it to vitamin K2.
Bacteria in the gut flora can also convert K1 into vitamin K2 (menaquinone). In addition, bacteria typically lengthen the isoprenoid side chain of vitamin K2 to produce a range of vitamin K2 forms, most notably the MK-7 to MK-11 homologues of vitamin K2. All forms of K2 other than MK-4 can only be produced by bacteria, which use these during anaerobic respiration. The MK-7 and other bacterially derived forms of vitamin K2 exhibit vitamin K activity in animals, but MK-7's extra utility over MK-4, if any, is unclear and is a matter of investigation.
Because a synthetic form of vitamin K, vitamin K3 (menadione), may be toxic by interfering with the function of glutathione, it is no longer used to treat vitamin K deficiency.[1]
Contents
1 Health effects
1.1 Osteoporosis
1.2 Cardiovascular health
1.3 Cancer
1.4 Warfarin overdose and coumarin poisoning
2 Side effects
3 Interactions
4 Chemistry
4.1 Conversion of vitamin K1 to vitamin K2
4.2 Vitamin K2
5 Physiology
6 Absorption and dietary need
7 Dietary recommendations
8 Food sources
8.1 Vitamin K1
8.2 Vitamin K2
9 Deficiency
10 Biochemistry
10.1 Function in animals
10.2 Gamma-carboxyglutamate proteins
10.3 Methods of assessment
10.4 Function in bacteria
11 Injection in newborns
11.1 United States
11.2 United Kingdom
11.3 Controversy
12 History
13 References
14 Bibliography
15 External links
Health effects[edit]
Osteoporosis[edit]
There is no good evidence that vitamin K supplementation benefits the bone health of postmenopausal women.[3]
Cardiovascular health[edit]
Adequate intake of vitamin K is associated with the inhibition of arterial calcification and stiffening,[4] but there have been few interventional studies and no good evidence that vitamin K supplementation is of any benefit in the primary prevention of cardiovascular disease.[5]
One 10-year population study, the Rotterdam Study, did show a clear and significant inverse relationship between the highest intake levels of menaquinone (mainly MK-4 from eggs and meat, and MK-8 and MK-9 from cheese) and cardiovascular disease and all-cause mortality in older men and women.[6]
Cancer[edit]
Vitamin K has been promoted in supplement form with claims it can slow tumor growth; however, no good medical evidence supports such claims.[7]
Warfarin overdose and coumarin poisoning[edit]
Vitamin K is one of the treatments for bleeding events caused by overdose of the anticoagulant drug warfarin (Coumadin®).[8] Vitamin K is also part of the suggested treatment regime for poisoning by rodenticide (coumarin poisoning).[9]
Side effects[edit]
Although allergic reaction from supplementation is possible, no known toxicity is associated with high doses of the phylloquinone (vitamin K1) or menaquinone (vitamin K2) forms of vitamin K, so no tolerable upper intake level (UL) has been set.[10]
Blood clotting (coagulation) studies in humans using 45 mg per day of vitamin K2 (as MK-4)[11] and even up to 135 mg per day (45 mg three times daily) of K2 (as MK-4),[12] showed no increase in blood clot risk. Even doses in rats as high as 250 mg/kg, body weight did not alter the tendency for blood-clot formation to occur.[13]
Unlike the safe natural forms of vitamin K1 and vitamin K2 and their various isomers, a synthetic form of vitamin K, vitamin K3 (menadione), is demonstrably toxic at high levels. The U.S. FDA has banned this form from over-the-counter sale in the United States because large doses have been shown to cause allergic reactions, hemolytic anemia, and cytotoxicity in liver cells.[1]
Interactions[edit]
Phylloquinone (K1)[14][15] or menaquinone (K2) are capable of reversing the anticoagulant activity of the anticoagulant warfarin (tradename Coumadin). Warfarin works by blocking recycling of vitamin K, so that the body and tissues have lower levels of active vitamin K, and thus a deficiency of vitamin K.
Supplemental vitamin K (for which oral dosing is often more active than injectable dosing in human adults) reverses the vitamin K deficiency caused by warfarin, and therefore reduces the intended anticoagulant action of warfarin and related drugs.[16] Sometimes small amounts of vitamin K are given orally to patients taking warfarin so that the action of the drug is more predictable.[16] The proper anticoagulant action of the drug is a function of vitamin K intake and drug dose, and due to differing absorption must be individualized for each patient.[citation needed] The action of warfarin and vitamin K both require two to five days after dosing to have maximum effect, and neither warfarin nor vitamin K shows much effect in the first 24 hours after they are given.[17]
The newer anticoagulants apixaban, dabigatran and rivaroxaban have different mechanisms of action that do not interact with vitamin K, and may be taken with supplemental vitamin K.[18][19]
Chemistry[edit]

Vitamin K2 (menaquinone). In menaquinone, the side chain is composed of a varying number of isoprenoid residues. The most common number of these residues is four, since animal enzymes normally produce menaquinone-4 from plant phylloquinone.
The structure of phylloquinone, Vitamin K1, is marked by the presence of a phytyl group.[20] The structures of menaquinones are marked by the polyisoprenyl side chain present in the molecule that can contain six to 13 isoprenyl units.[20]

A sample of phytomenadione for injection, also called phylloquinone
The three synthetic forms of vitamin K are vitamins K3 (menadione), K4, and K5, which are used in many areas, including the pet food industry (vitamin K3) and to inhibit fungal growth (vitamin K5).[21]
Conversion of vitamin K1 to vitamin K2[edit]

Vitamin K1 (phylloquinone) – both forms of the vitamin contain a functional naphthoquinone ring and an aliphatic side chain. Phylloquinone has a phytyl side chain.
The MK-4 form of vitamin K2 is produced by conversion of vitamin K1 in the testes, pancreas, and arterial walls.[22] While major questions still surround the biochemical pathway for this transformation, the conversion is not dependent on gut bacteria, as it occurs in germ-free rats[23][24] and in parenterally administered K1 in rats.[25][26] In fact, tissues that accumulate high amounts of MK-4 have a remarkable capacity to convert up to 90% of the available K1 into MK-4.[23][27] There is evidence that the conversion proceeds by removal of the phytyl tail of K1 to produce menadione as an intermediate, which is then condensed with an activated geranylgeranyl moiety (see also prenylation) to produce vitamin K2 in the MK-4 (menatetrenone) form.[28]
Vitamin K2[edit]
Vitamin K2 (menaquinone) includes several subtypes. The two most studied ones are menaquinone-4 (menatetrenone, MK-4) and menaquinone-7 (MK-7).
Physiology[edit]
Vitamin K1, the precursor of most vitamin K in nature, is a stereoisomer of phylloquinone, an important chemical in green plants, where it functions as an electron acceptor in photosystem I during photosynthesis. For this reason, vitamin K1 is found in large quantities in the photosynthetic tissues of plants (green leaves, and dark green leafy vegetables such as romaine lettuce, kale, and spinach), but it occurs in far smaller quantities in other plant tissues (roots, fruits, etc.). Iceberg lettuce contains relatively little. The function of phylloquinone in plants appears to have no resemblance to its later metabolic and biochemical function (as "vitamin K") in animals, where it performs a completely different biochemical reaction.
Vitamin K (in animals) is involved in the carboxylation of certain glutamate residues in proteins to form gamma-carboxyglutamate (Gla) residues. The modified residues are often (but not always) situated within specific protein domains called Gla domains. Gla residues are usually involved in binding calcium, and are essential for the biological activity of all known Gla proteins.[29]
At this time[update], 17 human proteins with Gla domains have been discovered, and they play key roles in the regulation of three physiological processes:
Blood coagulation: prothrombin (factor II), factors VII, IX, and X, and proteins C, S, and Z[30]
Bone metabolism: osteocalcin, also called bone Gla protein (BGP), matrix Gla protein (MGP),[31]periostin,[32] and the recently discovered Gla-rich protein (GRP).[33][34]
- Vascular biology: growth arrest-specific protein 6 (Gas6)[35]
- Unknown function: proline-rich γ-carboxyglutamyl proteins (PRGPs) 1 and 2, and transmembrane γ-carboxy glutamyl proteins (TMGs) 3 and 4.[36]
When Vitamin K1 enters the body through foods in a person's diet, it is absorbed through the jejunum and ileum in the small intestine[20], and like other lipid-soluble vitamins (A, D, and E), vitamin K is stored in the fatty tissue of the human body.
Absorption and dietary need[edit]
Previous theory held that dietary deficiency is extremely rare unless the small intestine was heavily damaged, resulting in malabsorption of the molecule. Another at-risk group for deficiency were those subject to decreased production of K2 by normal intestinal microbiota, as seen in broad-spectrum antibiotic use.[37] Taking broad-spectrum antibiotics can reduce vitamin K production in the gut by nearly 74% in people compared with those not taking these antibiotics.[38] Diets low in vitamin K also decrease the body's vitamin K concentration.[39] Those with chronic kidney disease are at risk for vitamin K deficiency, as well as vitamin D deficiency, and particularly those with the apoE4 genotype.[40] Additionally, the elderly have a reduction in vitamin K2.[41]
Dietary recommendations[edit]
The U.S. Institute of Medicine (IOM) updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for vitamin K in 1998. The IOM does not distinguish between K1 and K2 – both are counted as vitamin K. At that time, sufficient information was not available to establish EARs and RDAs for vitamin K. In instances such as these, the board sets Adequate Intakes (AIs), with the understanding that at some later date, AIs will be replaced by more exact information. The current AIs for adult women and men ages 19 and up are 90 and 120 μg/day, respectively. AI for pregnancy is 90 μg/day. AI for lactation is 90 μg/day. For infants up to 12 months, the AI is 2.0-2.5 μg/day; for children ages 1–18 years the AI increases with age from 30 to 75 μg/day. As for safety, the IOM sets tolerable upper intake levels (known as ULs) for vitamins and minerals when evidence is sufficient. Vitamin K has no UL, as human data for adverse effects from high doses are inadequate. Collectively, the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes.[20]
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL are defined the same as in United States. For women and men over age 18 the AI is set at 70 μg/day. AI for pregnancy is 70 μg/day, ad for lactation 70 μg/day. For children ages 1–17 years, the AIs increase with age from 12 to 65 μg/day. These AIs are lower than the U.S. RDAs.[42] The EFSA also reviewed the safety question and reached the same conclusion as in United States - that there was not sufficient evidence to set a UL for vitamin K.[43]
For U.S. food and dietary supplement labeling purposes, the amount in a serving is expressed as a percentage of Daily Value (%DV). For vitamin K labeling purposes, 100% of the Daily Value was 80 μg, but as of May 27, 2016, it was revised upwards to 120 μg, to bring it into agreement with the AI.[44] A table of the old and new adult Daily Values is provided at Reference Daily Intake. The original deadline to be in compliance was July 28, 2018, but on September 29, 2017, the FDA released a proposed rule that extended the deadline to January 1, 2020 for large companies and January 1, 2021 for small companies.[45]
Food sources[edit]
Vitamin K1[edit]
| Food | Serving size | Vitamin K1 (μg)[46] | Food | Serving size | Vitamin K1 (μg)[46] | |
|---|---|---|---|---|---|---|
Kale, cooked | 1⁄2 cup | 531 | Parsley, raw | 1⁄4 cup | 246 | |
Spinach, cooked | 1⁄2 cup, 77g | 444 | Spinach, raw | 1 cup | 145 | |
Collards, cooked | 1⁄2 cup | 418 | Collards, raw | 1 cup | 184 | |
Swiss chard, cooked | 1⁄2 cup | 287 | Swiss chard, raw | 1 cup | 299 | |
Mustard greens, cooked | 1⁄2 cup | 210 | Mustard greens, raw | 1 cup | 279 | |
Turnip greens, cooked | 1⁄2 cup | 265 | Turnip greens, raw | 1 cup | 138 | |
Broccoli, cooked | 1 cup | 220 | Broccoli, raw | 1 cup | 89 | |
Brussels sprouts, cooked | 1 cup | 219 | Endive, raw | 1 cup | 116 | |
Cabbage, cooked | 1⁄2 cup | 82 | Green leaf lettuce | 1 cup | 71 | |
Asparagus | 4 spears | 48 | Romaine lettuce, raw | 1 cup | 57 | |
Table from "Important information to know when you are taking: Warfarin (Coumadin) and Vitamin K", Clinical Center, National Institutes of Health Drug Nutrient Interaction Task Force.[47] | ||||||
Vitamin K1 is found chiefly in leafy green vegetables such as spinach, swiss chard, lettuce and Brassica vegetables (such as cabbage, kale, cauliflower, broccoli, and brussels sprouts) and often the absorption is greater when accompanied by fats such as butter or oils. Some fruits, such as avocados, kiwifruit and grapes, also contain vitamin K. Some vegetable oils, notably soybean oil, contain vitamin K, but at levels that would require relatively large calorie consumption to meet the recommended amounts.[48]
The tight binding of vitamin K1 to thylakoid membranes in chloroplasts makes it less bioavailable. For example, cooked spinach has a 5% bioavailability of phylloquinone, however, fat added to it increases bioavailability to 13% due to the increased solubility of vitamin K in fat.[49]
Vitamin K2[edit]
Vitamin K2, the form of generated by bacteria, can be found in eggs, dairy, and meat, as well as fermented foods such as cheese and yogurt.[4]
Deficiency[edit]
Average diets are usually not lacking in vitamin K, and primary deficiency is rare in healthy adults. Newborn infants are at an increased risk of deficiency. Other populations with an increased prevalence of vitamin K deficiency include those who suffer from liver damage or disease (e.g. alcoholics), cystic fibrosis, or inflammatory bowel diseases, or have recently had abdominal surgeries. Secondary vitamin K deficiency can occur in people with bulimia, those on stringent diets, and those taking anticoagulants. Other drugs associated with vitamin K deficiency include salicylates, barbiturates, and cefamandole, although the mechanisms are still unknown. Vitamin K deficiency has been defined as a vitamin K-responsive hypoprothrombinemia which increase prothrombin time[20] and thus can result in coagulopathy, a bleeding disorder.[50] Symptoms of K1 deficiency include anemia, bruising, nosebleeds and bleeding of the gums in both sexes, and heavy menstrual bleeding in women.
Osteoporosis[51][52] and coronary heart disease[53][54] are strongly associated with lower levels of K2 (menaquinone). Vitamin K2 (as menaquinones MK-4 through MK-10) intake level is inversely related to severe aortic calcification and all-cause mortality.[6]
Biochemistry[edit]
Function in animals[edit]

Mechanism of action of vitamin K1.
The function of vitamin K2 in the animal cell is to add a carboxylic acid functional group to a glutamate (Glu) amino acid residue in a protein, to form a gamma-carboxyglutamate (Gla) residue. This is a somewhat uncommon posttranslational modification of the protein, which is then known as a "Gla protein". The presence of two −COOH (carboxylic acid) groups on the same carbon in the gamma-carboxyglutamate residue allows it to chelate calcium ions. The binding of calcium ions in this way very often triggers the function or binding of Gla-protein enzymes, such as the so-called vitamin K-dependent clotting factors discussed below.
Within the cell, vitamin K undergoes electron reduction to a reduced form called vitamin K hydroquinone, catalyzed by the enzyme vitamin K epoxide reductase (VKOR).[55] Another enzyme then oxidizes vitamin K hydroquinone to allow carboxylation of Glu to Gla; this enzyme is called gamma-glutamyl carboxylase[56][57] or the vitamin K-dependent carboxylase. The carboxylation reaction only proceeds if the carboxylase enzyme is able to oxidize vitamin K hydroquinone to vitamin K epoxide at the same time. The carboxylation and epoxidation reactions are said to be coupled. Vitamin K epoxide is then reconverted to vitamin K by VKOR. The reduction and subsequent reoxidation of vitamin K coupled with carboxylation of Glu is called the vitamin K cycle.[58] Humans are rarely deficient in vitamin K1 because, in part, vitamin K1 is continuously recycled in cells.[59]
Warfarin and other 4-hydroxycoumarins block the action of VKOR.[60] This results in decreased concentrations of vitamin K and vitamin K hydroquinone in tissues, such that the carboxylation reaction catalyzed by the glutamyl carboxylase is inefficient. This results in the production of clotting factors with inadequate Gla. Without Gla on the amino termini of these factors, they no longer bind stably to the blood vessel endothelium and cannot activate clotting to allow formation of a clot during tissue injury. As it is impossible to predict what dose of warfarin will give the desired degree of clotting suppression, warfarin treatment must be carefully monitored to avoid overdose.
Gamma-carboxyglutamate proteins[edit]
The following human Gla-containing proteins ("Gla proteins") have been characterized to the level of primary structure: blood coagulation factors II (prothrombin), VII, IX, and X, anticoagulant protein C and protein S, and the factor X-targeting protein Z. The bone Gla protein osteocalcin, the calcification-inhibiting matrix Gla protein (MGP), the cell growth regulating growth arrest specific gene 6 protein (Gas6), and the four transmembrane Gla proteins (TMGPs), the function of which is at present unknown. Gas6 can function as a growth factor to activate the Axl receptor tyrosine kinase and stimulate cell proliferation or prevent apoptosis in some cells. In all cases in which their function was known, the presence of the Gla residues in these proteins turned out to be essential for functional activity.
Gla proteins are known to occur in a wide variety of vertebrates: mammals, birds, reptiles, and fish. The venom of a number of Australian snakes acts by activating the human blood-clotting system. In some cases, activation is accomplished by snake Gla-containing enzymes that bind to the endothelium of human blood vessels and catalyze the conversion of procoagulant clotting factors into activated ones, leading to unwanted and potentially deadly clotting.
Another interesting class of invertebrate Gla-containing proteins is synthesized by the fish-hunting snail Conus geographus.[61] These snails produce a venom containing hundreds of neuroactive peptides, or conotoxins, which is sufficiently toxic to kill an adult human. Several of the conotoxins contain two to five Gla residues.[62]
Methods of assessment[edit]
Vitamin K status can be assessed by:
- The prothrombin time (PT) test measures the time required for blood to clot. A blood sample is mixed with citric acid and put in a fibrometer; delayed clot formation indicates a deficiency. This test is insensitive to mild deficiency, as the values do not change until the concentration of prothrombin in the blood has declined by at least 50%.[63]
- Undercarboxylated prothrombin (PIVKA-II); in a study of 53 newborns, found "PT (prothrombin time) is a less sensitive marker than PIVKA II",[64] and as indicated above, PT is unable to detect subclinical deficiencies that can be detected with PIVKA-II testing.
- Plasma phylloquinone was found to be positively correlated with phylloquinone intake in elderly British women, but not men,[65] but an article by Schurgers et al. reported no correlation between responses in a food frequency questionnaire and plasma phylloquinone.[66]
- Urinary γ-carboxyglutamic acid responds to changes in dietary vitamin K intake. Several days are required before any change can be observed. In a study by Booth et al., increases of phylloquinone intakes from 100 μg to between 377 and 417 μg for five days did not induce a significant change. Response may be age-specific.[67]
- Undercarboxylated osteocalcin (UcOc) levels have been inversely correlated with stores of vitamin K[68] and bone strength in developing rat tibiae. Another study following 78 post-menopausal Korean women found a supplement regimen of vitamins K and D, and calcium, but not a regimen of vitamin D and calcium, was inversely correlated with reduced UcOc levels.[69]
Function in bacteria[edit]
Many bacteria, such as Escherichia coli found in the large intestine, can synthesize vitamin K2 (menaquinone-7 or MK-7, up to MK-11),[70] but not vitamin K1 (phylloquinone). In these bacteria, menaquinone transfers two electrons between two different small molecules, during oxygen-independent metabolic energy production processes (anaerobic respiration).[71] For example, a small molecule with an excess of electrons (also called an electron donor) such as lactate, formate, or NADH, with the help of an enzyme, passes two electrons to menaquinone. The menaquinone, with the help of another enzyme, then transfers these two electrons to a suitable oxidant, such fumarate or nitrate (also called an electron acceptor). Adding two electrons to fumarate or nitrate converts the molecule to succinate or nitrite plus water, respectively.
Some of these reactions generate a cellular energy source, ATP, in a manner similar to eukaryotic cell aerobic respiration, except the final electron acceptor is not molecular oxygen, but fumarate or nitrate. In aerobic respiration, the final oxidant is molecular oxygen (O2), which accepts four electrons from an electron donor such as NADH to be converted to water. E. coli, as facultative anaerobes, can carry out both aerobic respiration and menaquinone-mediated anaerobic respiration.
Injection in newborns[edit]
The blood clotting factors of newborn babies are roughly 30–60% that of adult values; this may be due to the reduced synthesis of precursor proteins and the sterility of their guts. Human milk contains 1–4 μg/L of vitamin K1, while formula-derived milk can contain up to 100 μg/L in supplemented formulas. Vitamin K2 concentrations in human milk appear to be much lower than those of vitamin K1. Occurrence of vitamin K deficiency bleeding in the first week of the infant's life is estimated at 0.25–1.7%, with a prevalence of 2–10 cases per 100,000 births.[72] Premature babies have even lower levels of the vitamin, so they are at a higher risk from this deficiency.
Bleeding in infants due to vitamin K deficiency can be severe, leading to hospitalization, blood transfusions, brain damage, and death. Supplementation can prevent most cases of vitamin K deficiency bleeding in the newborn. Intramuscular administration (known as the Vitamin K shot[73]) is more effective in preventing late vitamin K deficiency bleeding than oral administration.[74][75]
United States[edit]
As a result of the occurrences of vitamin K deficiency bleeding, the Committee on Nutrition of the American Academy of Pediatrics has recommended 0.5–1 mg of vitamin K1 be administered to all newborns shortly after birth.[74]
United Kingdom[edit]
In the UK, vitamin K supplementation is recommended for all newborns within the first 24 hours.[76] This is usually given as a single intramuscular injection of 1 mg shortly after birth but as a second-line option can be given by three oral doses over the first month.[77]
Controversy[edit]
Controversy arose in the early 1990s regarding this practice, when two studies suggested a relationship between parenteral administration of vitamin K and childhood cancer.[78] However, poor methods and small sample sizes led to the discrediting of these studies, and a review of the evidence published in 2000 by Ross and Davies found no link between the two.[79] Doctors reported emerging concerns in 2013,[80] after treating children for serious bleeding problems. They cited lack of newborn vitamin K administration as the reason that the problems occurred, and recommended that breastfed babies could have an increased risk unless they receive a preventative dose.
History[edit]
In 1929, Danish scientist Henrik Dam investigated the role of cholesterol by feeding chickens a cholesterol-depleted diet.[81] He initially replicated experiments reported by scientists at the Ontario Agricultural College (OAC).[82] McFarlane, Graham and Richardson, working on the chick feed program at OAC, had used chloroform to remove all fat from chick chow. They noticed that chicks fed only fat-depleted chow developed hemorrhages and started bleeding from tag sites.[83] Dam found that these defects could not be restored by adding purified cholesterol to the diet. It appeared that – together with the cholesterol – a second compound had been extracted from the food, and this compound was called the coagulation vitamin. The new vitamin received the letter K because the initial discoveries were reported in a German journal, in which it was designated as Koagulationsvitamin. Edward Adelbert Doisy of Saint Louis University did much of the research that led to the discovery of the structure and chemical nature of vitamin K.[84] Dam and Doisy shared the 1943 Nobel Prize for medicine for their work on vitamin K (K1 and K2) published in 1939. Several laboratories synthesized the compound(s) in 1939.[85]
For several decades, the vitamin K-deficient chick model was the only method of quantifying vitamin K in various foods: the chicks were made vitamin K-deficient and subsequently fed with known amounts of vitamin K-containing food. The extent to which blood coagulation was restored by the diet was taken as a measure for its vitamin K content. Three groups of physicians independently found this: Biochemical Institute, University of Copenhagen (Dam and Johannes Glavind), University of Iowa Department of Pathology (Emory Warner, Kenneth Brinkhous, and Harry Pratt Smith), and the Mayo Clinic (Hugh Butt, Albert Snell, and Arnold Osterberg).[86]
The first published report of successful treatment with vitamin K of life-threatening hemorrhage in a jaundiced patient with prothrombin deficiency was made in 1938 by Smith, Warner, and Brinkhous.[87]
The precise function of vitamin K was not discovered until 1974, when three laboratories (Stenflo et al.,[88] Nelsestuen et al.,[89] and Magnusson et al.[90]) isolated the vitamin K-dependent coagulation factor prothrombin (factor II) from cows that received a high dose of a vitamin K antagonist, warfarin. It was shown that, while warfarin-treated cows had a form of prothrombin that contained 10 glutamate (Glu) amino acid residues near the amino terminus of this protein, the normal (untreated) cows contained 10 unusual residues that were chemically identified as γ-carboxyglutamate (Gla). The extra carboxyl group in Gla made clear that vitamin K plays a role in a carboxylation reaction during which Glu is converted into Gla.
References[edit]
^ abcd "Vitamin K". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. July 2014. Retrieved 20 March 2017..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ "Vitamin K Overview". University of Maryland Medical Center.
^ Hamidi, M. S.; Gajic-Veljanoski, O.; Cheung, A. M. (2013). "Vitamin K and bone health". Journal of Clinical Densitometry (Review). 16 (4): 409–413. doi:10.1016/j.jocd.2013.08.017. PMID 24090644.
^ ab Maresz, K. (Feb 2015). "Proper Calcium Use: Vitamin K2 as a Promoter of Bone and Cardiovascular Health". Integrative Medicine (Review). 14 (1): 34–39. PMC 4566462. PMID 26770129.
^ Hartley, L.; Clar, C.; Ghannam, O.; Flowers, N.; Stranges, S.; Rees, K. (Sep 2015). "Vitamin K for the primary prevention of cardiovascular disease". The Cochrane Database of Systematic Reviews (Systematic review). 9 (9): CD011148. doi:10.1002/14651858.CD011148.pub2. PMID 26389791.
^ ab Geleijnse, J. M.; Vermeer, C.; Grobbee, D. E.; Schurgers, L. J.; Knapen, M. H.; van der Meer, I. M.; Hofman, A.; Witteman, J. C. (Nov 2004). "Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study". Journal of Nutrition. 134 (11): 3100–3105. doi:10.1093/jn/134.11.3100. PMID 15514282.
^ Ades, T. B., ed. (2009). "Vitamin K". American Cancer Society Complete Guide to Complementary and Alternative Cancer Therapies (2nd ed.). American Cancer Society. pp. 558–563. ISBN 978-0-944235-71-3.
^ Ageno, W; Gallus, AS; Wittkowsky, A; Crowther, M; Hylek, EM; Palareti, G; American College of Chest, Physicians. (February 2012). "Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines". Chest. 141 (2 Suppl): e44S–88S. doi:10.1378/chest.11-2292. PMC 3278051. PMID 22315269.
^ Lung, D. (Dec 2015). Tarabar, A., ed. "Rodenticide Toxicity Treatment & Management". Medscape. WebMD.
^ Rasmussen, S. E.; Andersen, N. L.; Dragsted, L. O.; Larsen, J. C. (Mar 2006). "A safe strategy for addition of vitamins and minerals to foods". European Journal of Nutrition. 45 (3): 123–135. doi:10.1007/s00394-005-0580-9. PMID 16200467.
^ Ushiroyama, T.; Ikeda, A.; Ueki, M (Mar 2002). "Effect of continuous combined therapy with vitamin K2 and vitamin D3 on bone mineral density and coagulofibrinolysis function in postmenopausal women". Maturitas. 41 (3): 211–221. doi:10.1016/S0378-5122(01)00275-4. PMID 11886767.
^ Asakura, H.; Myou, S.; Ontachi, Y.; Mizutani, T.; Kato, M.; Saito, M.; Morishita, E.; Yamazaki, M.; Nakao, S. (Dec 2001). "Vitamin K administration to elderly patients with osteoporosis induces no hemostatic activation, even in those with suspected vitamin K deficiency". Osteoporosis International. 12 (12): 996–1000. doi:10.1007/s001980170007. PMID 11846334.
^ Ronden, J. E.; Groenen-van Dooren, M. M.; Hornstra, G.; Vermeer, C. (Jul 1997). "Modulation of arterial thrombosis tendency in rats by vitamin K and its side chains". Atherosclerosis. 132 (1): 61–67. doi:10.1016/S0021-9150(97)00087-7. PMID 9247360.
^ Ansell, J.; Hirsh, J.; Poller, L.; Bussey, H.; Jacobson, A.; Hylek, E (Sep 2004). "The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy". Chest. 126 (3 Suppl): 204S–233S. doi:10.1378/chest.126.3_suppl.204S. PMID 15383473.
^ Crowther, M. A.; Douketis, J. D.; Schnurr, T.; Steidl, L.; Mera, V.; Ultori, C.; Venco, A.; Ageno, W. (Aug 2002). "Oral vitamin K lowers the international normalized ratio more rapidly than subcutaneous vitamin K in the treatment of warfarin-associated coagulopathy. A randomized, controlled trial". Annals of Internal Medicine. 137 (4): 251–254. doi:10.7326/0003-4819-137-4-200208200-00009. PMID 12186515.
^ ab "Important Information to Know When You Are Taking: Warfarin (Coumadin) and Vitamin K" (PDF). National Institute of Health Clinical Center Drug-Nutrient Interaction Task Force. Retrieved 17 Apr 2015.
^ "Guidelines For Warfarin Reversal With Vitamin K" (PDF). American Society of Health-System Pharmacists. Retrieved 17 Apr 2015.
^ "Pradaxa Drug Interactions". Pradaxapro.com. 10 July 2017. Retrieved 10 July 2017.
^ Bauersachs, R.; Berkowitz, S. D.; Brenner, B.; Buller, H. R.; Decousus, H.; Gallus, A. S.; Lensing, A. W.; Misselwitz, F.; Prins, M. H.; Raskob, G. E.; Segers, A.; Verhamme, P.; Wells, P.; Agnelli, G.; Bounameaux, H.; Cohen, A.; Davidson, B. L.; Piovella, F.; Schellong, S. (Dec 2010). "Oral rivaroxaban for symptomatic venous thromboembolism" (PDF). New England Journal of Medicine. 363 (26): 2499–2510. doi:10.1056/NEJMoa1007903. PMID 21128814.
^ abcde "Vitamin K". Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press. 2001. pp. 162–196.
^ McGee, W. (1 Feb 2007). "Vitamin K". MedlinePlus. Retrieved 2 Apr 2009.
^ Shearer, M. J.; Newman, P. (Oct 2008). "Metabolism and cell biology of vitamin K". Thrombosis and Haemostasis. 100 (4): 530–547. doi:10.1160/TH08-03-0147. PMID 18841274.
^ ab Davidson, R. T.; Foley, A. L.; Engelke, J. A.; Suttie, J. W. (Feb 1998). "Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria". Journal of Nutrition. 128 (2): 220–223. doi:10.1093/jn/128.2.220. PMID 9446847.
^ Ronden, J. E.; Drittij-Reijnders, M. J.; Vermeer, C.; Thijssen, H. H. (Jan 1998). "Intestinal flora is not an intermediate in the phylloquinone–menaquinone-4 conversion in the rat". Biochimica et Biophysica Acta. 1379 (1): 69–75. doi:10.1016/S0304-4165(97)00089-5. PMID 9468334.
^ Thijssen, H. .H.; Drittij-Reijnders, M. J. (Sep 1994). "Vitamin K distribution in rat tissues: dietary phylloquinone is a source of tissue menaquinone-4". The British Journal of Nutrition. 72 (3): 415–425. doi:10.1079/BJN19940043. PMID 7947656.
^ Will, B. H.; Usui, Y.; Suttie, J. W. (Dec 1992). "Comparative metabolism and requirement of vitamin K in chicks and rats". Journal of Nutrition. 122 (12): 2354–2360. doi:10.1093/jn/122.12.2354. PMID 1453219.
^ Ronden, J. E.; Drittij-Reijnders, M. J.; Vermeer, C.; Thijssen, H. H. (Jan 1998). "Intestinal flora is not an intermediate in the phylloquinone-menaquinone-4 conversion in the rat". Biochimica et Biophysica Acta. 1379 (1): 69–75. doi:10.1016/S0304-4165(97)00089-5. PMID 9468334.
^ Al Rajabi, Ala (2011). The Enzymatic Conversion of Phylloquinone to Menaquinone-4 (PhD thesis). Tufts University, Friedman School of Nutrition Science and Policy.
^ Furie, B.; Bouchard, B. A.; Furie, B. C. (Mar 1999). "Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid". Blood. 93 (6): 1798–1808. PMID 10068650.
^ Mann, K. G. (Aug 1999). "Biochemistry and physiology of blood coagulation". Thrombosis and Haemostasis. 82 (2): 165–174. doi:10.1055/s-0037-1615780. PMID 10605701.
^ Price, P. A. (1988). "Role of vitamin-K-dependent proteins in bone metabolism". Annual Review of Nutrition. 8: 565–583. doi:10.1146/annurev.nu.08.070188.003025. PMID 3060178.
^ Coutu, D. L.; Wu, J. H.; Monette, A.; Rivard, G. E.; Blostein, M. D.; Galipeau, J (Jun 2008). "Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells". Journal of Biological Chemistry. 283 (26): 17991–18001. doi:10.1074/jbc.M708029200. PMID 18450759.
^ Viegas, C. S.; Simes, D. C.; Laizé, V.; Williamson, M. K.; Price, P. A.; Cancela, M. L. (Dec 2008). "Gla-rich protein (GRP), a new vitamin K-dependent protein identified from sturgeon cartilage and highly conserved in vertebrates". Journal of Biological Chemistry. 283 (52): 36655–36664. doi:10.1074/jbc.M802761200. PMC 2605998. PMID 18836183.
^ Viegas, C. S.; Cavaco, S.; Neves, P. L.; Ferreira, A.; João, A.; Williamson, M. K.; Price, P. A.; Cancela, M. L.; Simes, D. C. (Dec 2009). "Gla-rich protein is a novel vitamin K-dependent protein present in serum that accumulates at sites of pathological calcifications". American Journal of Pathology. 175 (6): 2288–2298. doi:10.2353/ajpath.2009.090474. PMC 2789615. PMID 19893032.
^ Hafizi, S.; Dahlbäck, B. (Dec 2006). "Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily". The FEBS Journal. 273 (23): 5231–5244. doi:10.1111/j.1742-4658.2006.05529.x. PMID 17064312.
^ Kulman, J. D.; Harris, J. E.; Xie, L.; Davie, E. W. (May 2007). "Proline-rich Gla protein 2 is a cell-surface vitamin K-dependent protein that binds to the transcriptional coactivator Yes-associated protein". Proceedings of the National Academy of Sciences of the United States of America. 104 (21): 8767–8772. doi:10.1073/pnas.0703195104. PMC 1885577. PMID 17502622.
^ "Vitamin K". MedlinePlus. US National Library of Medicine, National Institutes of Health. Sep 2016. Retrieved 26 May 2009.
^ Conly, J; Stein, K. (Dec 1994). "Reduction of vitamin K2 concentrations in human liver associated with the use of broad spectrum antimicrobials". Clinical and Investigative Medicine. 17 (6): 531–539. PMID 7895417.
^ Ferland, G.; Sadowski, J. A.; O'Brien, M. E. (Apr 1993). "Dietary induced subclinical vitamin K deficiency in normal human subjects". Journal of Clinical Investigation. 91 (4): 1761–1768. doi:10.1172/JCI116386. PMC 288156. PMID 8473516.
^ Holden, R. M.; Morton, A. R.; Garland, J. S.; Pavlov, A.; Day, A. G.; Booth, S. L. (Apr 2010). "Vitamins K and D status in stages 3-5 chronic kidney disease". Clinical Journal of the American Society of Nephrology. 5 (4): 590–597. doi:10.2215/CJN.06420909. PMC 2849681. PMID 20167683.
^ Hodges, S. J.; Pilkington, M. J.; Shearer, M. J.; Bitensky, L.; Chayen, J (Jan 1990). "Age-related changes in the circulating levels of congeners of vitamin K2, menaquinone-7 and menaquinone-8". Clinical Science. 78 (1): 63–66. doi:10.1042/cs0780063. PMID 2153497.
^ "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies" (PDF). 2017.
^ "Tolerable Upper Intake Levels For Vitamins And Minerals" (PDF). European Food Safety Authority. 2006.
^ "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982" (PDF).
^ "Changes to the Nutrition Facts Panel - Compliance Date"
^ ab Rhéaume-Bleue, p. 42
^ "Important information to know when you are taking: Warfarin (Coumadin) and Vitamin K" (PDF). National Institutes of Health Clinical Center.
^ "Nutrition facts, calories in food, labels, nutritional information and analysis". Nutritiondata.com. 13 Feb 2008. Retrieved 21 Apr 2013.
^ "Vitamin K". Vivo.colostate.edu. 2 Jul 1999. Retrieved 21 Apr 2013.
^ "Vitamin K". Micronutrient Data Centre.
^ Ikeda, Y.; Iki, M.; Morita, A.; Kajita, E.; Kagamimori, S.; Kagawa, Y.; Yoneshima, H. (May 2006). "Intake of fermented soybeans, natto, is associated with reduced bone loss in postmenopausal women: Japanese Population-Based Osteoporosis (JPOS) Study". Journal of Nutrition. 136 (5): 1323–1328. doi:10.1093/jn/136.5.1323. PMID 16614424.
^ Katsuyama, H.; Ideguchi, S.; Fukunaga, M.; Saijoh, K.; Sunami, S. (Jun 2002). "Usual dietary intake of fermented soybeans (Natto) is associated with bone mineral density in premenopausal women". Journal of Nutritional Science and Vitaminology. 48 (3): 207–215. doi:10.3177/jnsv.48.207. PMID 12350079.
^ Sano, M.; Fujita, H.; Morita, I.; Uematsu, H.; Murota, S. (Dec 1999). "Vitamin K2 (menatetrenone) induces iNOS in bovine vascular smooth muscle cells: no relationship between nitric oxide production and gamma-carboxylation". Journal of Nutritional Science and Vitaminology. 45 (6): 711–723. doi:10.3177/jnsv.45.711. PMID 10737225.
^ Gast, G. C ; de Roos, N. M.; Sluijs, I.; Bots, M. L.; Beulens, J. W.; Geleijnse, J. M.; Witteman, J. C.; Grobbee, D. E.; Peeters, P. H.; van der Schouw, Y. T. (Sep 2009). "A high menaquinone intake reduces the incidence of coronary heart disease". Nutrition, Metabolism, and Cardiovascular Diseases. 19 (7): 504–510. doi:10.1016/j.numecd.2008.10.004. PMID 19179058.
^ Oldenburg, J.; Bevans, C. G.; Müller, C. R.; Watzka, M. (2006). "Vitamin K epoxide reductase complex subunit 1 (VKORC1): the key protein of the vitamin K cycle". Antioxidants & Redox Signaling. 8 (3–4): 347–353. doi:10.1089/ars.2006.8.347. PMID 16677080.
^ Suttie, J. W. (1985). "Vitamin K-dependent carboxylase". Annual Review of Biochemistry. 54: 459–477. doi:10.1146/annurev.bi.54.070185.002331. PMID 3896125.
^ Presnell, S. R.; Stafford, D. W. (Jun 2002). "The vitamin K-dependent carboxylase". Thrombosis and Haemostasis. 87 (6): 937–946. doi:10.1055/s-0037-1613115. PMID 12083499.
^ Stafford, D. W. (Aug 2005). "The vitamin K cycle". Journal of Thrombosis and Haemostasis. 3 (8): 1873–1878. doi:10.1111/j.1538-7836.2005.01419.x. PMID 16102054.
^ Rhéaume-Bleue, p. 79.
^ Whitlon, D. S.; Sadowski, J. A.; Suttie, J. W. (Apr 1978). "Mechanism of coumarin action: significance of vitamin K epoxide reductase inhibition". Biochemistry. 17 (8): 1371–1377. doi:10.1021/bi00601a003. PMID 646989.
^ Terlau, H.; Olivera, B. M. (Jan 2004). "Conus venoms: a rich source of novel ion channel-targeted peptides". Physiological Reviews. 84 (1): 41–68. doi:10.1152/physrev.00020.2003. PMID 14715910.
^ Buczek, O.; Bulaj, G.; Olivera, BM (Dec 2005). "Conotoxins and the posttranslational modification of secreted gene products". Cellular and Molecular Life Sciences. 62 (24): 3067–3079. doi:10.1007/s00018-005-5283-0. PMID 16314929.
^ "Prothrombin Time". WebMD.
^ Dituri, F.; Buonocore, G.; Pietravalle, A.; Naddeo, F.; Cortesi, M; Pasqualetti, P; Tataranno M. L.; R., Agostino (Sep 2012). "PIVKA-II plasma levels as markers of subclinical vitamin K deficiency in term infants". Journal of Maternal, Fetal & Neonatal Medicine. 25 (9): 1660–1663. doi:10.3109/14767058.2012.657273. PMID 22280352.
^ Thane, C. W.; Bates, C. J.; Shearer, M. J.; Unadkat, N; Harrington, D. J.; Paul, A. A.; Prentice, A.; Bolton-Smith, C. (Jun 2002). "Plasma phylloquinone (vitamin K1) concentration and its relationship to intake in a national sample of British elderly people". British Journal of Nutrition. 87 (6): 615–622. doi:10.1079/BJNBJN2002582. PMID 12067432.
^ McKeown, N. M.; Jacques, P. F.; Gundberg, C. M.; Peterson, J. W.; Tucker, K. L.; Kiel, D. P.; Wilson, P. W.; Booth, SL (Jun 2002). "Dietary and nondietary determinants of vitamin K biochemical measures in men and women" (PDF). Journal of Nutrition. 132 (6): 1329–1334. doi:10.1093/jn/132.6.1329. PMID 12042454.
^ Yamano, M.; Yamanaka, Y.; Yasunaga, K.; Uchida, K. (Sep 1989). "Effect of vitamin K deficiency on urinary gamma-carboxyglutamic acid excretion in rats". Nihon Ketsueki Gakkai Zasshi. 52 (6): 1078–1086. PMID 2588957.
^ Matsumoto, T.; Miyakawa, T.; Yamamoto, D. (Mar 2012). "Effects of vitamin K on the morphometric and material properties of bone in the tibiae of growing rats". Metabolism. 61 (3): 407–414. doi:10.1016/j.metabol.2011.07.018. PMID 21944271.
^ Je, S.-H.; Joo, N.-S.; Choi, B.-H.; Kim, K.-M.; Kim, B.-T.; Park, S.-B.; Cho, D.-Y.; Kim, K.-N.; Lee, D.-J. (Aug 2011). "Vitamin K supplement along with vitamin D and calcium reduced serum concentration of undercarboxylated osteocalcin while increasing bone mineral density in Korean postmenopausal women over sixty-years-old". Journal of Korean Medical Science. 26 (8): 1093–1098. doi:10.3346/jkms.2011.26.8.1093. PMC 3154347. PMID 21860562.
^ Bentley, R.; Meganathan, R. (Sep 1982). "Biosynthesis of vitamin K (menaquinone) in bacteria" (PDF). Microbiological Reviews. 46 (3): 241–280. PMC 281544. PMID 6127606.
^ Haddock, B. A.; Jones, C. W. (Mar 1977). "Bacterial respiration" (PDF). Bacteriological Reviews. 41 (1): 47–99. PMC 413996. PMID 140652.
^ Shearer, M. J. (Jan 1995). "Vitamin K". Lancet. 345 (8944): 229–234. doi:10.1016/S0140-6736(95)90227-9. PMID 7823718.
^ "Vitamin K Shot – Essential in Preventing Serious Bleeding in Newborns". www.cdc.gov. 2017-09-15. Retrieved 2018-07-06.
^ ab American Academy of Pediatrics Committee on Fetus Newborn. (2003). "Controversies concerning vitamin K and the newborn. American Academy of Pediatrics Committee on Fetus and Newborn" (PDF). Pediatrics. 112 (1.1): 191–192. doi:10.1542/peds.112.1.191. PMID 12837888.
^ Mihatsch WA, Braegger C, Bronsky J, Campoy C, Domellöf M, Fewtrell M, Mis NF, Hojsak I, Hulst J, Indrio F, Lapillonne A, Mlgaard C, Embleton N, van Goudoever J (2016). "Prevention of Vitamin K Deficiency Bleeding in Newborn Infants: A Position Paper by the ESPGHAN Committee on Nutrition". J. Pediatr. Gastroenterol. Nutr. 63 (1): 123–129. doi:10.1097/MPG.0000000000001232. PMID 27050049.
^ Logan, S.; Gilbert, R. (1998). "Vitamin K For Newborn Babies" (PDF). Department of Health. Archived from the original (PDF) on 7 January 2013. Retrieved 12 Oct 2014.
^ "Postnatal care: Routine postnatal care of women and their babies [CG37]". www.nice.org.uk. NICE. Jul 2006. Retrieved 12 Oct 2014.
^ Parker, L.; Cole, M.; Craft, A. W.; Hey, E. N. (1998). "Neonatal vitamin K administration and childhood cancer in the north of England: retrospective case-control study". BMJ (Clinical Research Ed.). 316 (7126): 189–193. doi:10.1136/bmj.316.7126.189. PMC 2665412. PMID 9468683.
^ McMillan, D. D. (1997). "Routine administration of vitamin K to newborns". Paediatric Child Health. 2 (6): 429–431. doi:10.1093/pch/2.6.429.
^ "Newborns get rare disorder after parents refused shots".Having four cases since February just at Vanderbilt was a little bit concerning to me
^ Dam, C. P. H. (1935). "The Antihaemorrhagic Vitamin of the Chick: Occurrence And Chemical Nature". Nature. 135 (3417): 652–653. doi:10.1038/135652b0.
^ Dam, C. P. H. (1941). "The discovery of vitamin K, its biological functions and therapeutical application" (PDF). Nobel Prize Laureate Lecture.
^ McAlister, V. C. (2006). "Control of coagulation: a gift of Canadian agriculture" (PDF). Clinical and Investigative Medicine. 29 (6): 373–377. Archived from the original (PDF) on 2010-03-06.
^ MacCorquodale, D. W.; Binkley, S. B.; Thayer, S. A.; Doisy, E. A. (1939). "On the constitution of Vitamin K1". Journal of the American Chemical Society. 61 (7): 1928–1929. doi:10.1021/ja01876a510.
^ Fieser, L. F. (1939). "Synthesis of Vitamin K1". Journal of the American Chemical Society. 61 (12): 3467–3475. doi:10.1021/ja01267a072.
^ Dam, C. P. H. (12 Dec 1946). "The discovery of vitamin K, its biological functions and therapeutical application" (PDF). Nobel Prize lecture.
^ Warner, E. D.; Brinkhous, K. M.; Smith, H. P. (1938). "Bleeding Tendency of Obstructive Jaundice". Proceedings of the Society for Experimental Biology and Medicine. 37 (4): 628–630. doi:10.3181/00379727-37-9668P.
^ Stenflo, J; Fernlund, P.; Egan, W.; Roepstorff, P. (Jul 1974). "Vitamin K dependent modifications of glutamic acid residues in prothrombin". Proceedings of the National Academy of Sciences of the United States of America. 71 (7): 2730–2733. doi:10.1073/pnas.71.7.2730. PMC 388542. PMID 4528109.
^ Nelsestuen, G. L.; Zytkovicz, T. H.; Howard, J. B. (Oct 1974). "The mode of action of vitamin K. Identification of gamma-carboxyglutamic acid as a component of prothrombin" (PDF). Journal of Biological Chemistry. 249 (19): 6347–6350. PMID 4214105.
^ Magnusson, S.; Sottrup-Jensen, L.; Petersen, T. E.; Morris, H. R.; Dell, A. (Aug 1974). "Primary structure of the vitamin K-dependent part of prothrombin". FEBS Letters. 44 (2): 189–193. doi:10.1016/0014-5793(74)80723-4. PMID 4472513.
Bibliography[edit]
Rhéaume-Bleue, Kate (2012). Vitamin K2 and the Calcium Paradox. John Wiley & Sons, Canada. ISBN 978-1-118-06572-3.
External links[edit]
"Vitamin K: Another Reason to Eat Your Greens".
Categories:
- Vitamin K
- Vitamins
- Antihemorrhagics
- 1,4-Naphthoquinones
- Terpenes and terpenoids
- Vitamers
(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgPageParseReport":{"limitreport":{"cputime":"1.172","walltime":"1.358","ppvisitednodes":{"value":5796,"limit":1000000},"ppgeneratednodes":{"value":0,"limit":1500000},"postexpandincludesize":{"value":260187,"limit":2097152},"templateargumentsize":{"value":2217,"limit":2097152},"expansiondepth":{"value":12,"limit":40},"expensivefunctioncount":{"value":7,"limit":500},"unstrip-depth":{"value":1,"limit":20},"unstrip-size":{"value":291826,"limit":5000000},"entityaccesscount":{"value":3,"limit":400},"timingprofile":["100.00% 1144.025 1 -total"," 66.32% 758.769 1 Template:Reflist"," 40.25% 460.469 63 Template:Cite_journal"," 14.56% 166.520 22 Template:Cite_web"," 6.79% 77.672 1 Template:Infobox_drug_class"," 6.28% 71.811 1 Template:Infobox"," 4.86% 55.642 1 Template:Citation_needed"," 4.72% 54.042 8 Template:Navbox"," 4.56% 52.130 1 Template:Fix"," 3.84% 43.907 1 Template:About"]},"scribunto":{"limitreport-timeusage":{"value":"0.692","limit":"10.000"},"limitreport-memusage":{"value":6884254,"limit":52428800},"limitreport-logs":"table#1 {n ["size"] = "tiny",n}n"},"cachereport":{"origin":"mw1263","timestamp":"20190313224153","ttl":2592000,"transientcontent":false}}});});{"@context":"https://schema.org","@type":"Article","name":"Vitamin K","url":"https://en.wikipedia.org/wiki/Vitamin_K","sameAs":"http://www.wikidata.org/entity/Q182338","mainEntity":"http://www.wikidata.org/entity/Q182338","author":{"@type":"Organization","name":"Contributors to Wikimedia projects"},"publisher":{"@type":"Organization","name":"Wikimedia Foundation, Inc.","logo":{"@type":"ImageObject","url":"https://www.wikimedia.org/static/images/wmf-hor-googpub.png"}},"datePublished":"2001-08-21T08:54:27Z","dateModified":"2019-03-12T20:55:42Z","image":"https://upload.wikimedia.org/wikipedia/commons/8/8e/Vitamin_K_structures.jpg","headline":"vitamin"}(window.RLQ=window.RLQ||).push(function(){mw.config.set({"wgBackendResponseTime":99,"wgHostname":"mw1330"});});