Membrane transport protein
A membrane transport protein (or simply transporter) is a membrane protein[1] involved in the movement of ions, small molecules, or macromolecules, such as another protein, across a biological membrane. Transport proteins are integral transmembrane proteins; that is they exist permanently within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion or active transport. The two main types of proteins involved in such transport are broadly categorized as either channels or carriers. The solute carriers and atypical SLCs[2] are secondary active or facilitative transporters in humans.[3][4]
Contents
1 Difference between channels and carriers
2 Active diffusion
3 Facilitated diffusion
4 Reverse diffusion
5 Types
5.1 1: Channels/pores
5.2 2: Electrochemical potential-driven transporters
5.3 3: Primary active transporters
5.4 4: Group translocators
5.5 5: Electron carriers
6 Examples
7 Pathology
8 See also
9 References
10 External links
Difference between channels and carriers
A carrier is not open simultaneously to both the extracellular and intracellular environments. Either its inner gate is open, or outer gate is open. In contrast, a channel can be open to both environments at the same time, allowing the molecules to diffuse without interruption. Carriers have binding sites, but pores and channels do not.[5][6][7] When a channel is opened, millions of ions can pass through the membrane per second, but only 100 to 1000 molecules typically pass through a carrier molecule in the same time.[8] Each carrier protein is designed to recognize only one substance or one group of very similar substances. Research has correlated defects in specific carrier proteins with specific diseases.[9]
Active diffusion

The action of the sodium-potassium pump is an example of primary active transport. The two carrier proteins on the left are using ATP to move sodium out of the cell against the concentration gradient. The proteins on the right are using secondary active transport to move potassium into the cell.
Active transport is the movement of a substance across a membrane against its concentration gradient. This is usually to accumulate high concentrations of molecules that a cell needs, such as glucose or amino acids. If the process uses chemical energy, such as adenosine triphosphate (ATP), it is called primary active transport. Secondary active transport involves the use of an electrochemical gradient, and does not use energy produced in the cell.[10] Unlike channel proteins which only transport substances through membranes passively, carrier proteins can transport ions and molecules either passively through facilitated diffusion, or via secondary active transport.[11] A carrier protein is required to move particles from areas of low concentration to areas of high concentration. These carrier proteins have receptors that bind to a specific molecule (substrate) needing transport. The molecule or ion to be transported (the substrate) must first bind at a binding site at the carrier molecule, with a certain binding affinity. Following binding, and while the binding site is facing the same way, the carrier will capture or occlude (take in and retain) the substrate within its molecular structure and cause an internal translocation so that the opening in the protein now faces the other side of the plasma membrane.[12] The carrier protein substrate is released at that site, according to its binding affinity there.
Facilitated diffusion
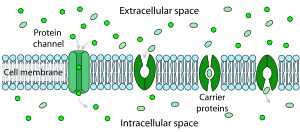
Facilitated diffusion in cell membrane, showing ion channels (left) and carrier proteins (three on the right).
Facilitated diffusion is the passage of molecules or ions across a biological membrane through specific transport proteins and requires no energy input. Facilitated diffusion is used especially in the case of large polar molecules and charged ions; once such ions are dissolved in water they cannot diffuse freely across cell membranes due to the hydrophobic nature of the fatty acid tails of the phospholipids that make up the bilayers.
The type of carrier proteins used in facilitated diffusion is slightly different from those used in active transport. They are still transmembrane carrier proteins, but these are gated transmembrane channels, meaning they do not internally translocate, nor require ATP to function. The substrate is taken in one side of the gated carrier, and without using ATP the substrate is released into the cell. They may be used as potential biomarkers
Reverse diffusion
Reverse transport, or transporter reversal, is a phenomenon in which the substrates of a membrane transport protein are moved in the opposite direction to that of their typical movement by the transporter.[13][14][15][16][17] Transporter reversal typically occurs when a membrane transport protein is phosphorylated by a particular protein kinase, which is an enzyme that adds a phosphate group to proteins.[13][14]
Types
(Grouped by Transporter Classification database categories)
1: Channels/pores
- α-helical protein channels such as voltage-gated ion channel (VIC), ligand-gated ion channels(LGICs)
- β-barrel porins such as aquaporin
- channel-forming toxins, including colicins, diphtheria toxin, and others
- Nonribosomally synthesized channels such as gramicidin
Holins; which function in export of enzymes that digest bacterial cell walls in an early step of cell lysis.
Facilitated diffusion occurs in and out of the cell membrane via channels/pores and carriers/porters.
Note:
- Channels:
Channels are either in open state or closed state. When a channel is opened with a slight conformational switch, it is open to both environment simultaneously (extracellular and intracellular)
Pores:

This picture represents symport. The yellow triangle shows the concentration gradient for the yellow circles while the green triangle shows the concentration gradient for the green circles and the purple rods are the transport protein bundle. The green circles are moving against their concentration gradient through a transport protein which requires energy while the yellow circles move down their concentration gradient which releases energy. The yellow circles produce more energy through chemiosmosis than what is required to move the green circles so the movement is coupled and some energy is cancelled out. One example is the lactose permease which allows protons to go down its concentration gradient into the cell while also pumping lactose into the cell.
Pores are continuously open to these both environment, because they do not undergo conformational changes. They are always open and active.
2: Electrochemical potential-driven transporters
- 2.A: Porters ( uniporters, symporters, antiporters), SLCs.[4]

The picture represents uniport. The yellow triangle shows the concentration gradient for the yellow circles and the purple rods are the transport protein bundle. Since they move down their concentration gradient through a transport protein, they can release energy as a result of chemiosmosis. One example is GLUT1 which moves glucose down its concentration gradient into the cell.
Excitatory amino acid transporters (EAATs)
- EAAT1
- EAAT2
- EAAT3
- EAAT4
- EAAT5
- Glucose transporter
Monoamine transporters, including:
Dopamine transporter (DAT)
Norepinephrine transporter (NET)
Serotonin transporter (SERT)
Vesicular monoamine transporters (VMAT)
Adenine nucleotide translocator (ANT)
- 2.B: Nonribosomally synthesized porters, such as
- The Nigericin (Nigericin) Family
- The Ionomycin (Ionomycin) Family
- 2.C: Ion-gradient-driven energizers
3: Primary active transporters
- 3.A: P-P-bond-hydrolysis-driven transporters :
ATP-binding cassette transporter (ABC transporter), such as MDR, CFTR
V-type ATPase ; ( "V" related to vacuolar ).
P-type ATPase ; ( "P" related to phosphorylation), such as :
- Na+/K+-ATPase
- Plasma membrane Ca2+ ATPase
- Proton pump

This picture represents antiport. The yellow triangle shows the concentration gradient for the yellow circles while the blue triangle shows the concentration gradient for the blue circles and the purple rods are the transport protein bundle. The blue circles are moving against their concentration gradient through a transport protein which requires energy while the yellow circles move down their concentration gradient which releases energy. The yellow circles produce more energy through chemiosmosis than what is required to move the blue circles so the movement is coupled and some energy is cancelled out. One example is the sodium-proton exchanger which allows protons to go down their concentration gradient into the cell while pumping sodium out of the cell.
F-type ATPase; ("F" related to factor), including: mitochondrial ATP synthase, chloroplast ATP synthase1
- 3.B: Decarboxylation-driven transporters
- 3.C: Methyltransfer-driven transporters
- 3.D: Oxidoreduction-driven transporters
- 3.E: Light absorption-driven transporters, such as rhodopsin
4: Group translocators
The group translocators provide a special mechanism for the phosphorylation of sugars as they are transported into bacteria (PEP group translocation)
5: Electron carriers
The transmembrane electron transfer carriers in the membrane include two-electron carriers, such as the disulfide bond oxidoreductases (DsbB and DsbD in E. coli) as well as one-electron carriers such as NADPH oxidase. Often these redox proteins are not considered transport proteins.
Examples
Each carrier protein, even within the same cell membrane, is specific to one type or family of molecules. For example, GLUT1 is a named carrier protein found in almost all animal cell membranes that transports glucose across the bilayer. Other specific carrier proteins also help the body function in important ways. Cytochromes operate in the electron transport chain as carrier proteins for electrons.[10]
Pathology
A number of inherited diseases involve defects in carrier proteins in a particular substance or group of cells. Cysteinuria (cysteine in the urine and the bladder) is such a disease involving defective cysteine carrier proteins in the kidney cell membranes. This transport system normally removes cysteine from the fluid destined to become urine and returns this essential amino acid to the blood. When this carrier malfunctions, large quantities of cysteine remain in the urine, where it is relatively insoluble and tends to precipitate. This is one cause of urinary stones.[18] Some vitamin carrier proteins have been shown to be overexpressed in patients with malignant disease. For example, levels of riboflavin carrier protein (RCP) have been shown to be significantly elevated in people with breast cancer.[19]
See also
- Cotransport
- Cotransporter
- Ion channel
- Permease
- P-loop
Solute carrier family (classification)
TC number (classification)- Translocase
- Vesicular transport protein
References
^ Membrane+transport+proteins at the US National Library of Medicine Medical Subject Headings (MeSH)
^ Perland, Emelie; Bagchi, Sonchita; Klaesson, Axel; Fredriksson, Robert (2017-09-01). "Characteristics of 29 novel atypical solute carriers of major facilitator superfamily type: evolutionary conservation, predicted structure and neuronal co-expression". Open Biology. 7 (9): 170142. doi:10.1098/rsob.170142. ISSN 2046-2441. PMC 5627054. PMID 28878041..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Hediger, Matthias A.; Romero, Michael F.; Peng, Ji-Bin; Rolfs, Andreas; Takanaga, Hitomi; Bruford, Elspeth A. (February 2004). "The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction". Pflügers Archiv: European Journal of Physiology. 447 (5): 465–468. doi:10.1007/s00424-003-1192-y. ISSN 0031-6768. PMID 14624363.
^ ab Perland, Emelie; Fredriksson, Robert (March 2017). "Classification Systems of Secondary Active Transporters". Trends in Pharmacological Sciences. 38 (3): 305–315. doi:10.1016/j.tips.2016.11.008. ISSN 1873-3735. PMID 27939446.
^ Sadava, David, et al. Life, the Science of Biology, 9th Edition. Macmillan Publishers, 2009.
ISBN 1-4292-1962-9. p. 119.
^ Cooper, Geoffrey (2009). The Cell: A Molecular Approach. Washington, DC: ASM Press. p. 62. ISBN 9780878933006.
^ Thompson, Liz A. Passing the North Carolina End of Course Test for Biology. American Book Company, Inc. 2007.
ISBN 1-59807-139-4. p. 97.
^ Assmann, Sarah (2015). "Solute Transport". In Taiz, Lincoln; Zeiger, Edward. Plant Physiology and Development. Sinauer. p. 151.
^ Sadava, David, Et al. Life, the Science of Biology, 9th Edition. Macmillan Publishers, 2009.
ISBN 1-4292-1962-9. p. 119.
^ ab Ashley, Ruth. Hann, Gary. Han, Seong S. Cell Biology. New Age International Publishers.
ISBN 8122413978. p. 113.
^ Taiz, Lincoln. Zeigler, Eduardo. Plant Physiology and Development. Sinauer Associates, 2015.
ISBN 978-1-60535-255-8. pp. 151.
^ Kent, Michael. Advanced Biology. Oxford University Press US, 2000.
ISBN 0-19-914195-9. pp. 157–158.
^ ab Bermingham DP, Blakely RD (October 2016). "Kinase-dependent Regulation of Monoamine Neurotransmitter Transporters". Pharmacol. Rev. 68 (4): 888–953. doi:10.1124/pr.115.012260. PMC 5050440. PMID 27591044.
^ ab Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
^ Scholze P, Nørregaard L, Singer EA, Freissmuth M, Gether U, Sitte HH (2002). "The role of zinc ions in reverse transport mediated by monoamine transporters". The Journal of Biological Chemistry. 277 (24): 21505–13. doi:10.1074/jbc.M112265200. PMID 11940571.
^ Robertson SD, Matthies HJ, Galli A (2009). "A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters". Molecular Neurobiology. 39 (2): 73–80. doi:10.1007/s12035-009-8053-4. PMC 2729543. PMID 19199083.
^ Kasatkina LA, Borisova TA (November 2013). "Glutamate release from platelets: exocytosis versus glutamate transporter reversal". The International Journal of Biochemistry & Cell Biology. 45 (11): 2585–2595. doi:10.1016/j.biocel.2013.08.004. PMID 23994539.
^ Sherwood, Lauralee. 7th Edition. Human Physiology. From Cells to Systems. Cengage Learning, 2008. p. 67
^ Rao, PN, Levine, E et al. Elevation of Serum Riboflavin Carrier Protein in Breast Cancer. Cancer Epidemiol Biomarkers Prev. Volume 8 No 11. pp. 985–990
External links
| Wikimedia Commons has media related to Membrane transport proteins. |
- "Transport protein" at Dorland's Medical Dictionary
DDI Regulatory Guidance Request a guide to drug-drug interaction regulatory recommendations.


