Glia
| Glia | |
|---|---|
 Illustration of the four different types of glial cells found in the central nervous system: ependymal cells (light pink), astrocytes (green), microglial cells (dark red), and oligodendrocytes (light blue). | |
| Details | |
| Precursor | Neuroectoderm for macroglia, and hematopoietic stem cells for microglia |
| System | Nervous system |
| Identifiers | |
| MeSH | D009457 |
| TA | A14.0.00.005 |
| TH | H2.00.06.2.00001 |
| FMA | 54541 |
Anatomical terms of microanatomy [edit on Wikidata] | |
Glia, also called glial cells or neuroglia, are non-neuronal cells in the central nervous system (brain and spinal cord) and the peripheral nervous system. They maintain homeostasis, form myelin, and provide support and protection for neurons.[1] In the central nervous system, glial cells include oligodendrocytes, astrocytes, ependymal cells, and microglia, and in the peripheral nervous system glial cells include Schwann cells and satellite cells. They have four main functions: (1) to surround neurons and hold them in place; (2) to supply nutrients and oxygen to neurons; (3) to insulate one neuron from another; (4) to destroy pathogens and remove dead neurons. They also play a role in neurotransmission and synaptic connections,[2] and in physiological processes like breathing.[3][4]
Glia were discovered in 1856, by the pathologist Rudolf Virchow in his search for a "connective tissue" in the brain.[5] The term derives from Greek γλία and γλοία "glue"(/ˈɡliːə/ or /ˈɡlaɪə/), and suggests the original impression that they were the glue of the nervous system.
Contents
1 Types
1.1 Macroglia
1.2 Microglia
1.3 Other
1.4 Total number
2 Development
2.1 Capacity to divide
3 Functions
3.1 Neuron repair and development
3.2 Myelin sheath creation
3.3 Neurotransmission
4 Clinical significance
5 History
6 See also
7 References
7.1 Bibliography
8 Further reading
9 External links
Types

Neuroglia of the brain shown by Golgi's method
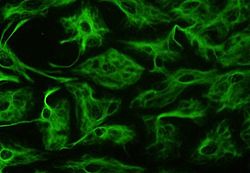
Astrocytes can be identified in culture because, unlike other mature glia, they express glial fibrillary acidic protein (GFAP)

Glial cells in a rat brain stained with an antibody against GFAP
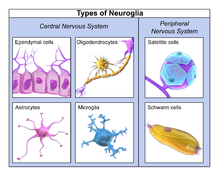
Different types of neuroglia
Macroglia
Derived from ectodermal tissue.
| Location | Name | Description |
|---|---|---|
| CNS | Astrocytes | The most abundant type of macroglial cell in the CNS,[6]astrocytes (also called astroglia) have numerous projections that link neurons to their blood supply while forming the blood-brain barrier. They regulate the external chemical environment of neurons by removing excess potassium ions, and recycling neurotransmitters released during synaptic transmission. Astrocytes may regulate vasoconstriction and vasodilation by producing substances such as arachidonic acid, whose metabolites are vasoactive. Astrocytes signal each other using ATP. The gap junctions (also known as electrical synapses) between astrocytes allow the messenger molecule IP3 to diffuse from one astrocyte to another. IP3 activates calcium channels on cellular organelles, releasing calcium into the cytoplasm. This calcium may stimulate the production of more IP3 and cause release of ATP through channels in the membrane made of pannexins. The net effect is a calcium wave that propagates from cell to cell. Extracellular release of ATP, and consequent activation of purinergic receptors on other astrocytes, may also mediate calcium waves in some cases. In general, there are two types of astrocytes, protoplasmic and fibrous, similar in function but distinct in morphology and distribution. Protoplasmic astrocytes have short, thick, highly branched processes and are typically found in gray matter. Fibrous astrocytes have long, thin, less branched processes and are more commonly found in white matter. It has recently been shown that astrocyte activity is linked to blood flow in the brain, and that this is what is actually being measured in fMRI.[7] They also have been involved in neuronal circuits playing an inhibitory role after sensing changes in extracellular calcium.[8] |
| CNS | Oligodendrocytes | Oligodendrocytes are cells that coat axons in the central nervous system (CNS) with their cell membrane, forming a specialized membrane differentiation called myelin, producing the myelin sheath. The myelin sheath provides insulation to the axon that allows electrical signals to propagate more efficiently.[9] |
| CNS | Ependymal cells | Ependymal cells, also named ependymocytes, line the spinal cord and the ventricular system of the brain. These cells are involved in the creation and secretion of cerebrospinal fluid (CSF) and beat their cilia to help circulate the CSF and make up the blood-CSF barrier. They are also thought to act as neural stem cells.[10] |
| CNS | Radial glia | Radial glia cells arise from neuroepithelial cells after the onset of neurogenesis. Their differentiation abilities are more restricted than those of neuroepithelial cells. In the developing nervous system, radial glia function both as neuronal progenitors and as a scaffold upon which newborn neurons migrate. In the mature brain, the cerebellum and retina retain characteristic radial glial cells. In the cerebellum, these are Bergmann glia, which regulate synaptic plasticity. In the retina, the radial Müller cell is the glial cell that spans the thickness of the retina and, in addition to astroglial cells,[11] participates in a bidirectional communication with neurons.[12] |
| PNS | Schwann cells | Similar in function to oligodendrocytes, Schwann cells provide myelination to axons in the peripheral nervous system (PNS). They also have phagocytotic activity and clear cellular debris that allows for regrowth of PNS neurons.[13] |
| PNS | Satellite cells | Satellite glial cells are small cells that surround neurons in sensory, sympathetic, and parasympathetic ganglia.[14] These cells help regulate the external chemical environment. Like astrocytes, they are interconnected by gap junctions and respond to ATP by elevating intracellular concentration of calcium ions. They are highly sensitive to injury and inflammation, and appear to contribute to pathological states, such as chronic pain.[15] |
| PNS | Enteric glial cells | Are found in the intrinsic ganglia of the digestive system. They are thought to have many roles in the enteric system, some related to homeostasis and muscular digestive processes.[16] |
Microglia
Microglia are specialized macrophages capable of phagocytosis that protect neurons of the central nervous system.[17] They are derived from the earliest wave of mononuclear cells that originate in yolk sac blood islands early in development, and colonize the brain shortly after the neural precursors begin to differentiate.[18]
These cells are found in all regions of the brain and spinal cord. Microglial cells are small relative to macroglial cells, with changing shapes and oblong nuclei. They are mobile within the brain and multiply when the brain is damaged. In the healthy central nervous system, microglia processes constantly sample all aspects of their environment (neurons, macroglia and blood vessels). In a healthy brain, microglia direct the immune response to brain damage and play an important role in the inflammation that accompanies the damage. Many diseases and disorders are associated with deficient microglia, such as Alzheimer's disease, Parkinson's disease, and ALS.
Other
Pituicytes from the posterior pituitary are glia cells with characteristics in common to astrocytes.[19]Tanycytes in the median eminence of the hypothalamus are a type of ependymal cell that descend from radial glia and line the base of the third ventricle.[20]
Total number
In general, neuroglial cells are smaller than neurons. There are approximately 85 billion glia cells in the human brain,[21] about the same number as neurons.[21] Glial cells make up about half the total volume of the brain and spinal cord.[22] The glia to neuron-ratio varies from one part of the brain to another. The glia to neuron-ratio in the cerebral cortex is 3.72 (60.84 billion glia (72%); 16.34 billion neurons), while that of the cerebellum is only 0.23 (16.04 billion glia; 69.03 billion neurons). The ratio in the cerebral cortex gray matter is 1.48, with 3.76 for the gray and white matter combined.[22] The ratio of the basal ganglia, diencephalon and brainstem combined is 11.35.[22]
The total number of glia cells in the human brain is distributed into the different types with oligodendrocytes being the most frequent (45–75%), followed by astrocytes (19–40%) and microglia (about 10% or less).[21]
Development

23-week fetal brain culture astrocyte
Most glia are derived from ectodermal tissue of the developing embryo, in particular the neural tube and crest. The exception is microglia, which are derived from hemopoietic stem cells. In the adult, microglia are largely a self-renewing population and are distinct from macrophages and monocytes, which infiltrate the injured and diseased CNS.
In the central nervous system, glia develop from the ventricular zone of the neural tube. These glia include the oligodendrocytes, ependymal cells, and astrocytes. In the peripheral nervous system, glia derive from the neural crest. These PNS glia include Schwann cells in nerves and satellite glial cells in ganglia.
Current research involving glial cells in the human cochlea proposes that these cells are the common precursor to both mature Schwann cells and satellite glial cells. Additionally, the peripheral glial cells located along the peripheral processes expressed NGFR, indicating a phenotype distinct from the peripheral glial cells located along the central processes.[23]
Capacity to divide
Glia retain the ability to undergo cell division in adulthood, whereas most neurons cannot. The view is based on the general deficiency of the mature nervous system in replacing neurons after an injury, such as a stroke or trauma, while very often there is a profound proliferation of glia, or gliosis near or at the site of damage. However, detailed studies found no evidence that 'mature' glia, such as astrocytes or oligodendrocytes, retain the ability of mitosis. Only the resident oligodendrocyte precursor cells seem to keep this ability after the nervous system matures. On the other hand, there are a few regions in the mature nervous system, such as the dentate gyrus of the hippocampus and the subventricular zone, where generation of new neurons can be observed.[24]
Glial cells are known to be capable of mitosis. By contrast, scientific understanding of whether neurons are permanently post-mitotic,[25] or capable of mitosis,[26][27][28] is still developing. In the past, glia had been considered[by whom?] to lack certain features of neurons. For example, glial cells were not believed to have chemical synapses or to release transmitters. They were considered to be the passive bystanders of neural transmission. However, recent studies have shown this to be untrue.[29]
Functions
Some glial cells function primarily as the physical support for neurons. Others regulate the internal environment of the brain, especially the fluid surrounding neurons and their synapses, and nutrify neurons. During early embryogenesis, glial cells direct the migration of neurons and produce molecules that modify the growth of axons and dendrites.
Neuron repair and development
Glia are also crucial in the development of the nervous system and in processes such as synaptic plasticity and synaptogenesis. Glia have a role in the regulation of repair of neurons after injury. In the central nervous system (CNS), glia suppress repair. Glial cells known as astrocytes enlarge and proliferate to form a scar and produce inhibitory molecules that inhibit regrowth of a damaged or severed axon. In the peripheral nervous system (PNS), glial cells known as Schwann cells promote repair. After axonal injury, Schwann cells regress to an earlier developmental state to encourage regrowth of the axon. This difference between the CNS and the PNS, raises hopes for the regeneration of nervous tissue in the CNS. For example, a spinal cord may be able to be repaired following injury or severance. Schwann cells are also known as neuri-lemmocytes. These cells envelop nerve fibers of the PNS by winding repeatedly around a nerve fiber with the nucleus inside of it. This process creates a myelin sheath, which not only aids in conductivity but also assists in the regeneration of damaged fibers.
Myelin sheath creation
Oligodendrocytes are another type of glial cell of the CNS. These dendrocytes resemble an octopus bulbous body and contain up to fifteen arm-like processes. Each “arm” reaches out to a nerve fiber and spirals around it, creating a myelin sheath. This myelin sheath insulates the nerve fiber from the extracellular fluid as well as speeds up the signal conduction in the nerve fiber.[30]
Neurotransmission
Recent research indicates that glial cells of the hippocampus and cerebellum participate in synaptic transmission, regulate the clearance of neurotransmitters from the synaptic cleft, and release gliotransmitters such as ATP, which modulate synaptic function.[31]
Astrocytes are crucial in clearance of neurotransmitters from within the synaptic cleft, which provides distinction between arrival of action potentials and prevents toxic build-up of certain neurotransmitters such as glutamate (excitotoxicity). It is also thought that glia play a role in many neurological diseases, including Alzheimer's disease.[32] Furthermore, at least in vitro, astrocytes can release gliotransmitter glutamate in response to certain stimulation. Another unique type of glial cell, the oligodendrocyte precursor cells or OPCs, have very well-defined and functional synapses from at least two major groups of neurons.[33] The only notable differences between neurons and glial cells are neurons' possession of axons and dendrites, and capacity to generate action potentials.
Clinical significance

Neoplastic glial cells stained with an antibody against GFAP (brown), from a brain biopsy
While glial cells in the PNS frequently assist in regeneration of lost neural functioning, loss of neurons in the CNS does not result in a similar reaction from neuroglia.[13] In the CNS, regrowth will only happen if the trauma was mild, and not severe.[34] When severe trauma presents itself, the survival of the remaining neurons becomes the optimal solution. However, some studies investigating the role of glial cells in Alzheimer's Disease are beginning to contradict the usefulness of this feature, and even claim it can "exacerbate" the disease.[35] In addition to impacting the potential repair of neurons in Alzheimer's Disease, scarring and inflammation from glial cells have been further implicated in the degeneration of neurons caused by Amyotrophic lateral sclerosis.[36]
In addition to neurodegenerative diseases, a wide range of harmful exposure, such as hypoxia, or physical trauma, can lead to the end result of physical damage to the CNS.[34] Generally, when damage occurs to the CNS, glial cells cause Apoptosis among the surrounding cellular bodies.[34] Then, there is a large amount of microglial activity, which results in inflammation, and finally, there is a heavy release of growth inhibiting molecules.[34]
History
Glia were first described in 1856 by the pathologist Rudolf Virchow in a comment to his 1846 publication on connective tissue. A more detailed description of glial cells was provided in the 1858 book Cellular Pathology by the same author.[37]
When markers for different types of cells were analyzed, Albert Einstein's brain was discovered to contain significantly more glia than normal brains in the left angular gyrus, an area thought to be responsible for mathematical processing and language.[38]
The ratio of glia to neurons increases with our definition of intelligence. Not only does the ratio of glia to neurons increase through evolution, but so does the size of the glia. Astroglial cells in the human have a volume 27 times greater than the same cells in the mouse's brain.[39]
These important scientific findings may begin to shift the neuron-specific perspective into a more holistic view of the brain which encompasses the glial cells as well. The glia's importance is becoming ever more clear as time goes on and new research is conducted. For most of the last century, scientists had written off glial cells as being nothing more than the structure and foundations that hold the neurons in place. But now, there is direct evidence that correlates the number of glial cells in the brain with the amount of intelligence that any given species possesses.[40] Future research will begin to shed light on the mysterious, yet increasingly crucial, role of glial cells.
See also
- Polydendrocytes
- List of human cell types derived from the germ layers
References
^ Jessen KR, Mirsky R (August 1980). "Glial cells in the enteric nervous system contain glial fibrillary acidic protein". Nature. 286 (5774): 736–7. doi:10.1038/286736a0. PMID 6997753..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Wolosker H, Dumin E, Balan L, Foltyn VN (July 2008). "D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration". The FEBS Journal. 275 (14): 3514–26. doi:10.1111/j.1742-4658.2008.06515.x. PMID 18564180.
^ Swaminathan, Nikhil (Jan–Feb 2011). "Glia—the other brain cells". Discover.
^ Gourine AV, Kasymov V, Marina N, et al. (July 2010). "Astrocytes control breathing through pH-dependent release of ATP". Science. 329 (5991): 571–5. doi:10.1126/science.1190721. PMC 3160742. PMID 20647426.
^ "Classic Papers". Network Glia. Max Delbrueck Center für Molekulare Medizin (MDC) Berlin-Buch. Retrieved 14 November 2015.
^ http://www.scientificamerican.com/article.cfm?id=the-root-of-thought-what[full citation needed]
^ Swaminathan N (2008). "Brain-scan mystery solved". Scientific American Mind. Oct–Nov: 7.
^ Torres A (2012). "Extracellular Ca2+ Acts as a Mediator of Communication from Neurons to Glia". Science Signaling. 5 Jan 24: 208. doi:10.1126/scisignal.2002160. PMC 3548660.
^ Baumann N, Pham-Dinh D (April 2001). "Biology of oligodendrocyte and myelin in the mammalian central nervous system". Physiological Reviews. 81 (2): 871–927. doi:10.1152/physrev.2001.81.2.871. PMID 11274346.CS1 maint: Uses authors parameter (link)
^ Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J (January 1999). "Identification of a neural stem cell in the adult mammalian central nervous system". Cell. 96 (1): 25–34. doi:10.1016/S0092-8674(00)80956-3. PMID 9989494.CS1 maint: Uses authors parameter (link)
^ Newman EA (October 2003). "New roles for astrocytes: regulation of synaptic transmission". Trends in Neurosciences. 26 (10): 536–42. doi:10.1016/S0166-2236(03)00237-6. PMID 14522146.
^ Campbell K, Götz M (May 2002). "Radial glia: multi-purpose cells for vertebrate brain development". Trends in Neurosciences. 25 (5): 235–8. doi:10.1016/s0166-2236(02)02156-2. PMID 11972958.CS1 maint: Uses authors parameter (link)
^ ab Jessen KR, Mirsky R (September 2005). "The origin and development of glial cells in peripheral nerves". Nature Reviews. Neuroscience. 6 (9): 671–82. doi:10.1038/nrn1746. PMID 16136171.CS1 maint: Uses authors parameter (link)
^ Hanani, M. Satellite glial cells in sensory ganglia: from form to function. Brain Res. Rev. 48:457–476, 2005
^ Ohara PT, Vit JP, Bhargava A, Jasmin L (December 2008). "Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo". Journal of Neurophysiology. 100 (6): 3064–73. doi:10.1152/jn.90722.2008. PMC 2604845. PMID 18715894.CS1 maint: Uses authors parameter (link)
^ Bassotti G, Villanacci V, Antonelli E, Morelli A, Salerni B (July 2007). "Enteric glial cells: new players in gastrointestinal motility?". Laboratory Investigation. 87 (7): 628–32. doi:10.1038/labinvest.3700564. PMID 17483847.CS1 maint: Uses authors parameter (link)
^ Brodal, 2010: p. 19
^ Never-resting microglia: physiological roles in the healthy brain and pathological implications
A Sierra, ME Tremblay, H Wake - 2015 - books.google.com
^ Miyata, S; Furuya, K; Nakai, S; Bun, H; Kiyohara, T (April 1999). "Morphological plasticity and rearrangement of cytoskeletons in pituicytes cultured from adult rat neurohypophysis". Neuroscience research. 33 (4): 299–306. doi:10.1016/s0168-0102(99)00021-8. PMID 10401983.
^ Rodríguez, EM; Blázquez, JL; Pastor, FE; Peláez, B; Peña, P; Peruzzo, B; Amat, P (2005). "Hypothalamic tanycytes: a key component of brain-endocrine interaction". International review of cytology. 247: 89–164. doi:10.1016/s0074-7696(05)47003-5. PMID 16344112.
^ abc von Bartheld, Christopher S.; Bahney, Jami; Herculano-Houzel, Suzana (2016-12-15). "The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting". The Journal of Comparative Neurology. 524 (18): 3865–3895. doi:10.1002/cne.24040. ISSN 1096-9861. PMC 5063692. PMID 27187682.
^ abc Azevedo FA, Carvalho LR, Grinberg LT, et al. (April 2009). "Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain". The Journal of Comparative Neurology. 513 (5): 532–41. doi:10.1002/cne.21974. PMID 19226510.
^ Locher H, de Groot JC, van Iperen L, Huisman MA, Frijns JH, Chuva de Sousa Lopes SM (2014). "Distribution and development of peripheral glial cells in the human fetal cochlea". PLoS ONE. 9 (1): e88066. doi:10.1371/journal.pone.0088066. PMC 3909285. PMID 24498246.
^ Kornack DR, Rakic P (May 1999). "Continuation of neurogenesis in the hippocampus of the adult macaque monkey". Proceedings of the National Academy of Sciences of the United States of America. 96 (10): 5768–73. doi:10.1073/pnas.96.10.5768. PMC 21935. PMID 10318959.
^ Herrup K, Yang Y (May 2007). "Cell cycle regulation in the postmitotic neuron: oxymoron or new biology?". Nature Reviews. Neuroscience. 8 (5): 368–78. doi:10.1038/nrn2124. PMID 17453017.
^ Goldman SA, Nottebohm F (April 1983). "Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain". Proceedings of the National Academy of Sciences of the United States of America. 80 (8): 2390–4. doi:10.1073/pnas.80.8.2390. PMC 393826. PMID 6572982.
^ Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. (November 1998). "Neurogenesis in the adult human hippocampus". Nature Medicine. 4 (11): 1313–7. doi:10.1038/3305. PMID 9809557.
^ Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E (April 1999). "Hippocampal neurogenesis in adult Old World primates". Proceedings of the National Academy of Sciences of the United States of America. 96 (9): 5263–7. doi:10.1073/pnas.96.9.5263. PMC 21852. PMID 10220454.
^ The Other Brain, by R. Douglas Fields, Ph. D. Simon & Schuster, 2009[page needed]
^ Saladin, Kenneth. Anatomy and Physiology, 6th Edition. McGraw Hill 2012. Page 446-448.
^ Newman, Eric A. (2003). "New roles for astrocytes: Regulation of synaptic transmission". Trends in Neurosciences. 26: 536–542. doi:10.1016/S0166-2236(03)00237-6. PMID 14522146.
^ Sadigh-Eteghad S, Majdi A, Mahmoudi J, Golzari SE, Talebi M (June 2016). "Astrocytic and microglial nicotinic acetylcholine receptors: an overlooked issue in Alzheimer's disease". Journal of Neural Transmission. doi:10.1007/s00702-016-1580-z. PMID 27262818.
^ Feezel, Charlie. World Cocoa Foundation: Knowledge Creation in Rulal West Africa. US Aid Education Workshop.[page needed]
^ abcd Puves, Dale (2012). Neuroscience 5th Ed. Sinauer Associates. pp. 560–580. ISBN 978-0878936465.
^ Lopategui Cabezas, I.; Batista, A. Herrera; Rol, G. Pentón (2014). "Papel de la glía en la enfermedad de Alzheimer. Futuras implicaciones terapéuticas". Neurología. 29 (5): 305–309. doi:10.1016/j.nrl.2012.10.006.
^ Valori, Chiara F.; Brambilla, Liliana; Martorana, Francesca; Rossi, Daniela (2013-08-03). "The multifaceted role of glial cells in amyotrophic lateral sclerosis". Cellular and Molecular Life Sciences. 71 (2): 287–297. doi:10.1007/s00018-013-1429-7. ISSN 1420-682X.
^ Kettenmann H, Verkhratsky A (December 2008). "Neuroglia: the 150 years after". Trends in Neurosciences. 31 (12): 653–9. doi:10.1016/j.tins.2008.09.003. PMID 18945498.
^ Diamond MC, Scheibel AB, Murphy GM Jr, Harvey T,"On the Brain of a Scientist: Albert Einstein","Experimental Neurology 1985;198-204", Retrieved February 18, 2017
^ Koob, Andrew (2009). The Root of Thought. FT Press. p. 186. ISBN 978-0-13-715171-4.
^ Aw, B.L. "5 Reasons why Glial Cells Were So Critical to Human Intelligence". Scientific Brains. Retrieved 5 January 2015.
Bibliography
Brodal, Per (2010). "Glia". The central nervous system: structure and function. Oxford University Press. p. 19. ISBN 978-0-19-538115-3.
- Kettenmann and Ransom, Neuroglia, Oxford University Press, 2012,
ISBN 978-0-19-979459-1 |http://ukcatalogue.oup.com/product/9780199794591.do#.UVcswaD3Ay4%7C
Puves, Dale (2012). Neuroscience 5th Ed. Sinauer Associates. pp. 560–580. ISBN 978-0878936465.
Further reading
Barres BA (November 2008). "The mystery and magic of glia: a perspective on their roles in health and disease". Neuron. 60 (3): 430–40. doi:10.1016/j.neuron.2008.10.013. PMID 18995817.
- Role of glia in synapse development
Overstreet LS (February 2005). "Quantal transmission: not just for neurons". Trends in Neurosciences. 28 (2): 59–62. doi:10.1016/j.tins.2004.11.010. PMID 15667925.
Peters A (May 2004). "A fourth type of neuroglial cell in the adult central nervous system". Journal of Neurocytology. 33 (3): 345–57. doi:10.1023/B:NEUR.0000044195.64009.27. PMID 15475689.
Volterra A, Steinhäuser C (August 2004). "Glial modulation of synaptic transmission in the hippocampus". Glia. 47 (3): 249–57. doi:10.1002/glia.20080. PMID 15252814.
Huang YH, Bergles DE (June 2004). "Glutamate transporters bring competition to the synapse". Current Opinion in Neurobiology. 14 (3): 346–52. doi:10.1016/j.conb.2004.05.007. PMID 15194115.
Artist ADSkyler(uses concepts of neuroscience and found inspiration from Glia)
External links
| Wikimedia Commons has media related to Glia. |
- Audio
"The Other Brain"—The Leonard Lopate Show (WNYC) "Neuroscientist Douglas Field, explains how glia, which make up approximately 85 percent of the cells in the brain, work. In The Other Brain: From Dementia to Schizophrenia, How New Discoveries about the Brain Are Revolutionizing Medicine and Science, he explains recent discoveries in glia research and looks at what breakthroughs in brain science and medicine are likely to come."
"Network Glia" A homepage devoted to glial cells.